
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.
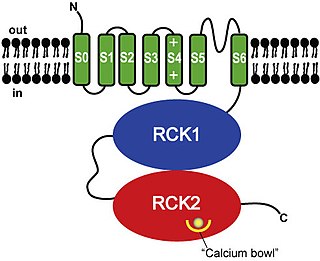
BK channels (big potassium), are large conductance calcium-activated potassium channels, also known as Maxi-K, slo1, or Kca1.1. BK channels are voltage-gated potassium channels that conduct large amounts of potassium ions (K+) across the cell membrane, hence their name, big potassium. These channels can be activated (opened) by either electrical means, or by increasing Ca2+ concentrations in the cell. BK channels help regulate physiological processes, such as circadian behavioral rhythms and neuronal excitability. BK channels are also involved in many processes in the body, as it is a ubiquitous channel. They have a tetrameric structure that is composed of a transmembrane domain, voltage sensing domain, potassium channel domain, and a cytoplasmic C-terminal domain, with many X-ray structures for reference. Their function is to repolarize the membrane potential by allowing for potassium to flow outward, in response to a depolarization or increase in calcium levels.

In biology, a connexon, also known as a connexin hemichannel, is an assembly of six proteins called connexins that form the pore for a gap junction between the cytoplasm of two adjacent cells. This channel allows for bidirectional flow of ions and signaling molecules. The connexon is the hemichannel supplied by a cell on one side of the junction; two connexons from opposing cells normally come together to form the complete intercellular gap junction channel. In some cells, the hemichannel itself is active as a conduit between the cytoplasm and the extracellular space, allowing the transference of ions and small molecules lower than 1-2 KDa. Little is known about this function of connexons besides the new evidence suggesting their key role in intracellular signaling. In still other cells connexons have been shown to occur in mitochondrial membranes and appear to play a role in heart ischaemia.
Calcium release-activated channels (CRAC) are specialized plasma membrane Ca2+ ion channels. When calcium ions (Ca2+) are depleted from the endoplasmic reticulum (a major store of Ca2+) of mammalian cells, the CRAC channel is activated to slowly replenish the level of calcium in the endoplasmic reticulum. The Ca2+ Release-activated Ca2+ (CRAC) Channel (CRAC-C) Family (TC# 1.A.52) is a member of the Cation Diffusion Facilitator (CDF) Superfamily. These proteins typically have between 4 and 6 transmembrane α-helical spanners (TMSs). The 4 TMS CRAC channels arose by loss of 2TMSs from 6TMS CDF carriers, an example of 'reverse' evolution'.
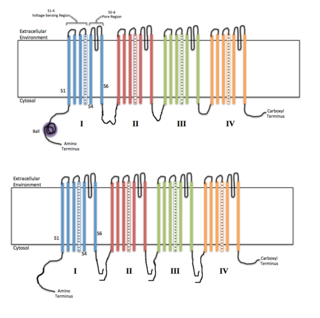
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in a cell's electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels.

Chloride channels are a superfamily of poorly understood ion channels specific for chloride. These channels may conduct many different ions, but are named for chloride because its concentration in vivo is much higher than other anions. Several families of voltage-gated channels and ligand-gated channels have been characterized in humans.
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g. muscle, glial cells, neurons) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+–Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions.

Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels.

The P2X receptors, also ATP-gated P2X receptor cation channel family, is a protein family that consists of cation-permeable ligand-gated ion channels that open in response to the binding of extracellular adenosine 5'-triphosphate (ATP). They belong to a larger family of receptors known as the ENaC/P2X superfamily. ENaC and P2X receptors have similar 3-D structures and are homologous. P2X receptors are present in a diverse array of organisms including humans, mouse, rat, rabbit, chicken, zebrafish, bullfrog, fluke, and amoeba.
Calcium-activated potassium channels are potassium channels gated by calcium, or that are structurally or phylogenetically related to calcium gated channels. They were first discovered in 1958 by Gardos who saw that calcium levels inside of a cell could affect the permeability of potassium through that cell membrane. Then in 1970, Meech was the first to observe that intracellular calcium could trigger potassium currents. In humans they are divided into three subtypes: large conductance or BK channels, which have very high conductance which range from 100 to 300 pS, intermediate conductance or IK channels, with intermediate conductance ranging from 25 to 100 pS, and small conductance or SK channels with small conductances from 2-25 pS.
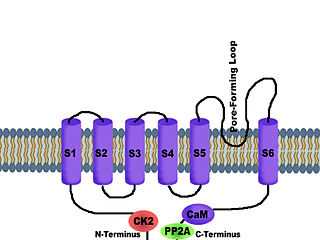
SK channels are a subfamily of calcium-activated potassium channels. They are so called because of their small single channel conductance in the order of 10 pS. SK channels are a type of ion channel allowing potassium cations to cross the cell membrane and are activated (opened) by an increase in the concentration of intracellular calcium through N-type calcium channels. Their activation limits the firing frequency of action potentials and is important for regulating afterhyperpolarization in the neurons of the central nervous system as well as many other types of electrically excitable cells. This is accomplished through the hyperpolarizing leak of positively charged potassium ions along their concentration gradient into the extracellular space. This hyperpolarization causes the membrane potential to become more negative. SK channels are thought to be involved in synaptic plasticity and therefore play important roles in learning and memory.

Nicotinic acid adenine dinucleotide phosphate (NAADP) is a Ca2+-mobilizing second messenger synthesised in response to extracellular stimuli. Like its mechanistic cousins, IP3 and cyclic adenosine diphosphoribose (Cyclic ADP-ribose), NAADP binds to and opens Ca2+ channels on intracellular organelles, thereby increasing the intracellular Ca2+ concentration which, in turn, modulates sundry cellular processes (see Calcium signalling). Structurally, it is a dinucleotide that only differs from the house-keeping enzyme cofactor, NADP by a hydroxyl group (replacing the nicotinamide amino group) and yet this minor modification converts it into the most potent Ca2+-mobilizing second messenger yet described. NAADP acts across phyla from plants to humans.
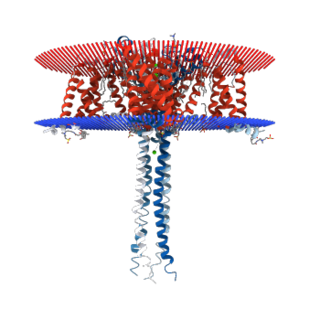
N-type calcium channels, also called Cav2.2 channels, are voltage gated calcium channels that are localized primarily on the nerve terminals and dendrites as well as neuroendocrine cells. The calcium N-channel consists of several subunits: the primary subunit α1B and the auxiliary subunits α2δ and β. The α1B subunit forms the pore through which the calcium enters and helps to determine most of the channel's properties. These channels play an important role in the neurotransmission during development. In the adult nervous system, N-type calcium channels are critically involved in the release of neurotransmitters, and in pain pathways. N-type calcium channels are the target of ziconotide, the drug prescribed to relieve intractable cancer pain. There are many known N-type calcium channel blockers that function to inhibit channel activity, although the most notable blockers are ω-conotoxins.

The L-type calcium channel is part of the high-voltage activated family of voltage-dependent calcium channel. "L" stands for long-lasting referring to the length of activation. This channel has four isoforms: Cav1.1, Cav1.2, Cav1.3, and Cav1.4.
Calcium-activated potassium channel subunit beta-2 is a protein that in humans is encoded by the KCNMB2 gene.

Two pore segment channel 2 (TPC2) is a protein which in humans is encoded by the TPCN2 gene. TPC2 is an ion channel, however, in contrast to other calcium and sodium channels which have four homologous domains, each containing 6 transmembrane segments, TPCN1 only contains two domain.
Vesicle fusion is the merging of a vesicle with other vesicles or a part of a cell membrane. In the latter case, it is the end stage of secretion from secretory vesicles, where their contents are expelled from the cell through exocytosis. Vesicles can also fuse with other target cell compartments, such as a lysosome. Exocytosis occurs when secretory vesicles transiently dock and fuse at the base of cup-shaped structures at the cell plasma membrane called porosome, the universal secretory machinery in cells. Vesicle fusion may depend on SNARE proteins in the presence of increased intracellular calcium (Ca2+) concentration.
In electrophysiology, the term gating refers to the opening (activation) or closing of ion channels. This change in conformation is a response to changes in transmembrane voltage.
The insulin transduction pathway is a biochemical pathway by which insulin increases the uptake of glucose into fat and muscle cells and reduces the synthesis of glucose in the liver and hence is involved in maintaining glucose homeostasis. This pathway is also influenced by fed versus fasting states, stress levels, and a variety of other hormones.
A calcium channel opener is a type of drug which facilitates ion transmission through calcium channels.














