Inositol 1,4,5-trisphosphate receptor, type 3, also known as ITPR3, is a protein which in humans is encoded by the ITPR3 gene. [5] The protein encoded by this gene is both a receptor for inositol triphosphate and a calcium channel. [6]
Inositol 1,4,5-trisphosphate receptor, type 3, also known as ITPR3, is a protein which in humans is encoded by the ITPR3 gene. [5] The protein encoded by this gene is both a receptor for inositol triphosphate and a calcium channel. [6]
ITP3 channels serve an important role in the taste transduction pathway of sweet, bitter and umami tastes the gustatory system. ITP3 channels allow the flow of Calcium out of the endoplasmic reticulum in response to IP3. Calcium cations result in the activation of TRPM5 which leads to a depolarisation generating potential and an action potential. [7]
Inositol trisphosphate or inositol 1,4,5-trisphosphate abbreviated InsP3 or Ins3P or IP3 is an inositol phosphate signaling molecule. It is made by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, by phospholipase C (PLC). Together with diacylglycerol (DAG), IP3 is a second messenger molecule used in signal transduction in biological cells. While DAG stays inside the membrane, IP3 is soluble and diffuses through the cell, where it binds to its receptor, which is a calcium channel located in the endoplasmic reticulum. When IP3 binds its receptor, calcium is released into the cytosol, thereby activating various calcium regulated intracellular signals.

Inositol trisphosphate receptor (InsP3R) is a membrane glycoprotein complex acting as a Ca2+ channel activated by inositol trisphosphate (InsP3). InsP3R is very diverse among organisms, and is necessary for the control of cellular and physiological processes including cell division, cell proliferation, apoptosis, fertilization, development, behavior, learning and memory. Inositol triphosphate receptor represents a dominant second messenger leading to the release of Ca2+ from intracellular store sites. There is strong evidence suggesting that the InsP3R plays an important role in the conversion of external stimuli to intracellular Ca2+ signals characterized by complex patterns relative to both space and time, such as Ca2+ waves and oscillations.
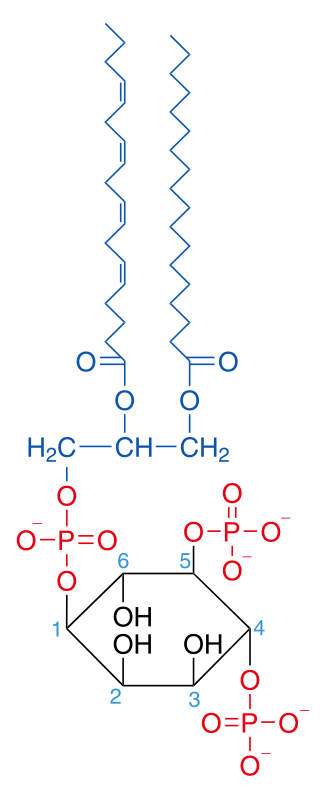
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins. PIP2 also forms lipid clusters that sort proteins.

Inositol-trisphosphate 3-kinase B is an enzyme that in humans is encoded by the ITPKB gene.

Inositol 1,4,5-trisphosphate receptor type 1 is a protein that in humans is encoded by the ITPR1 gene.

Inositol (1,4,5) trisphosphate 3-kinase (EC 2.7.1.127), abbreviated here as ITP3K, is an enzyme that facilitates a phospho-group transfer from adenosine triphosphate to 1D-myo-inositol 1,4,5-trisphosphate. This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:1D-myo-inositol-1,4,5-trisphosphate 3-phosphotransferase. ITP3K catalyzes the transfer of the gamma-phosphate from ATP to the 3-position of inositol 1,4,5-trisphosphate to form inositol 1,3,4,5-tetrakisphosphate. ITP3K is highly specific for the 1,4,5-isomer of IP3, and it exclusively phosphorylates the 3-OH position, producing Ins(1,3,4,5)P4, also known as inositol tetrakisphosphate or IP4.

1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase beta-2 is an enzyme that in humans is encoded by the PLCB2 gene.

1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase beta-3 is an enzyme that in humans is encoded by the PLCB3 gene.

1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase delta-1 is an enzyme that in humans is encoded by the PLCD1 gene. PLCd1 is essential to maintain homeostasis of the skin.

Inositol-trisphosphate 3-kinase A is an enzyme that in humans is encoded by the ITPKA gene.

Calcium binding protein 1 is a protein that in humans is encoded by the CABP1 gene. Calcium-binding protein 1 is a calcium-binding protein discovered in 1999. It has two EF hand motifs and is expressed in neuronal cells in such areas as hippocampus, habenular nucleus of the epithalamus, Purkinje cell layer of the cerebellum, and the amacrine cells and cone bipolar cells of the retina.

Putative adenosylhomocysteinase 2 is an enzyme that in humans is encoded by the AHCYL1 gene.

Ras GTPase-activating protein 3 is an enzyme that in humans is encoded by the RASA3 gene.

Protein MRVI1 is a protein that in humans is encoded by the MRVI1 gene.

Type I inositol-1,4,5-trisphosphate 5-phosphatase is an enzyme that in humans is encoded by the INPP5A gene.

Inositol-tetrakisphosphate 1-kinase is an enzyme that in humans is encoded by the ITPK1 gene.

Ankyrin 1, also known as ANK-1, and erythrocyte ankyrin, is a protein that in humans is encoded by the ANK1 gene.

Inositol 1,4,5-trisphosphate receptor, type 2, also known as ITPR2, is a protein which in humans is encoded by the ITPR2 gene. The protein encoded by this gene is both a receptor for inositol triphosphate and a calcium channel.

Ryanodine receptor 3 is one of a class of ryanodine receptors and a protein that in humans is encoded by the RYR3 gene. The protein encoded by this gene is both a calcium channel and a receptor for the plant alkaloid ryanodine. RYR3 and RYR1 control the resting calcium ion concentration in skeletal muscle.
The ryanodine-inositol 1,4,5-triphosphate receptor Ca2+ channel (RIR-CaC) family includes Ryanodine receptors and Inositol trisphosphate receptors. Members of this family are large proteins, some exceeding 5000 amino acyl residues in length. This family belongs to the Voltage-gated ion channel (VIC) superfamily. Ry receptors occur primarily in muscle cell sarcoplasmic reticular (SR) membranes, and IP3 receptors occur primarily in brain cell endoplasmic reticular (ER) membranes where they effect release of Ca2+ into the cytoplasm upon activation (opening) of the channel. They are redox sensors, possibly providing a partial explanation for how they control cytoplasmic Ca2+. Ry receptors have been identified in heart mitochondria where they provide the main pathway for Ca2+ entry. Sun et al. (2011) have demonstrated oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel (RyR1;TC# 1.A.3.1.2) by NADPH oxidase 4.