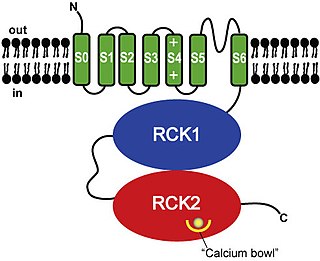
BK channels (big potassium), are large conductance calcium-activated potassium channels, also known as Maxi-K, slo1, or Kca1.1. BK channels are voltage-gated potassium channels that conduct large amounts of potassium ions (K+) across the cell membrane, hence their name, big potassium. These channels can be activated (opened) by either electrical means, or by increasing Ca2+ concentrations in the cell. BK channels help regulate physiological processes, such as circadian behavioral rhythms and neuronal excitability. BK channels are also involved in many processes in the body, as it is a ubiquitous channel. They have a tetrameric structure that is composed of a transmembrane domain, voltage sensing domain, potassium channel domain, and a cytoplasmic C-terminal domain, with many X-ray structures for reference. Their function is to repolarize the membrane potential by allowing for potassium to flow outward, in response to a depolarization or increase in calcium levels.
Calcium-activated potassium channels are potassium channels gated by calcium, or that are structurally or phylogenetically related to calcium gated channels. They were first discovered in 1958 by Gardos who saw that calcium levels inside of a cell could affect the permeability of potassium through that cell membrane. Then in 1970, Meech was the first to observe that intracellular calcium could trigger potassium currents. In humans they are divided into three subtypes: large conductance or BK channels, which have very high conductance which range from 100 to 300 pS, intermediate conductance or IK channels, with intermediate conductance ranging from 25 to 100 pS, and small conductance or SK channels with small conductances from 2-25 pS.

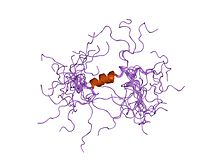
Calcium-activated potassium channel subunit alpha-1 also known as large conductance calcium-activated potassium channel, subfamily M, alpha member 1 (KCa1.1), or BK channel alpha subunit, is a voltage gated potassium channel encoded by the KCNMA1 gene and characterized by their large conductance of potassium ions (K+) through cell membranes.

Potassium voltage-gated channel subfamily D member 2 is a protein that in humans is encoded by the KCND2 gene. It contributes to the cardiac transient outward potassium current (Ito1), the main contributing current to the repolarizing phase 1 of the cardiac action potential.

Potassium intermediate/small conductance calcium-activated channel, subfamily N, member 4, also known as KCNN4, is a human gene encoding the KCa3.1 protein.
Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 is a protein that in humans is encoded by the GNG2 gene.

Calcium-activated potassium channel subunit beta-1 is a protein that in humans is encoded by the KCNMB1 gene.

Chloride channel accessory 1 is a protein that in humans is encoded by the CLCA1 gene.

Cav1.1 also known as the calcium channel, voltage-dependent, L type, alpha 1S subunit, (CACNA1S), is a protein which in humans is encoded by the CACNA1S gene. It is also known as CACNL1A3 and the dihydropyridine receptor.

Potassium voltage-gated channel, Shab-related subfamily, member 1, also known as KCNB1 or Kv2.1, is a protein that, in humans, is encoded by the KCNB1 gene.

G protein-activated inward rectifier potassium channel 1(GIRK-1) is encoded in the human by the gene KCNJ3.

Voltage-dependent L-type calcium channel subunit beta-4 is a protein that in humans is encoded by the CACNB4 gene.

Voltage-gated potassium channel subunit beta-1 is a protein that in humans is encoded by the KCNAB1 gene.

Voltage-dependent L-type calcium channel subunit beta-3 is a protein that in humans is encoded by the CACNB3 gene.

Calcium-activated potassium channel subunit beta-3 is a protein that in humans is encoded by the KCNMB3 gene.

Potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2, also known as KCNN2, is a protein which in humans is encoded by the KCNN2 gene. KCNN2 is an ion channel protein also known as KCa2.2.

Potassium voltage-gated channel subfamily KQT member 5 is a protein that in humans is encoded by the KCNQ5 gene.

Calcium-activated potassium channel subunit beta-4 is a protein that in humans is encoded by the KCNMB4 gene.

Potassium channel, subfamily U, member 1, also known as KCNU1, is a gene encoding the KCa5.1 protein.

In neuroscience, ball and chain inactivation is a model to explain the fast inactivation mechanism of voltage-gated ion channels. The process is also called hinged-lid inactivation or N-type inactivation. A voltage-gated ion channel can be in three states: open, closed, or inactivated. The inactivated state is mainly achieved through fast inactivation, by which a channel transitions rapidly from an open to an inactivated state. The model proposes that the inactivated state, which is stable and non-conducting, is caused by the physical blockage of the pore. The blockage is caused by a "ball" of amino acids connected to the main protein by a string of residues on the cytoplasmic side of the membrane. The ball enters the open channel and binds to the hydrophobic inner vestibule within the channel. This blockage causes inactivation of the channel by stopping the flow of ions. This phenomenon has mainly been studied in potassium channels and sodium channels.