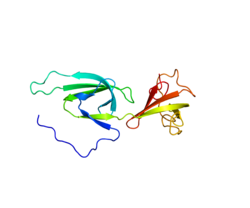
Eukaryotic translation initiation factor 5A-1 is a protein that in humans is encoded by the EIF5A gene. [5]
Contents
It is the only known protein to contain the unusual amino acid hypusine [Nε-(4-amino-2-hydroxybutyl)-lysine], which is synthesized on eIF5A at a specific lysine residue from the polyamine spermidine by two catalytic steps. [6]
EF-P is the bacterial homolog of eIF5A, which is modified post-translationally in a similar but distinct way. [7] [8] Both proteins are believed to catalyze peptide bond formation and help resolve ribosomal stalls, making them elongation factors despite the "initiation factor" name originally assigned. [9]