
Haloperidol, sold under the brand name Haldol among others, is a typical antipsychotic medication. Haloperidol is used in the treatment of schizophrenia, tics in Tourette syndrome, mania in bipolar disorder, delirium, agitation, acute psychosis, and hallucinations from alcohol withdrawal. It may be used by mouth or injection into a muscle or a vein. Haloperidol typically works within 30 to 60 minutes. A long-acting formulation may be used as an injection every four weeks for people with schizophrenia or related illnesses, who either forget or refuse to take the medication by mouth.

The atypical antipsychotics (AAP), also known as second generation antipsychotics (SGAs) and serotonin–dopamine antagonists (SDAs), are a group of antipsychotic drugs largely introduced after the 1970s and used to treat psychiatric conditions. Some atypical antipsychotics have received regulatory approval for schizophrenia, bipolar disorder, irritability in autism, and as an adjunct in major depressive disorder.

A dopamine antagonist, also known as an anti-dopaminergic and a dopamine receptor antagonist (DRA), is a type of drug which blocks dopamine receptors by receptor antagonism. Most antipsychotics are dopamine antagonists, and as such they have found use in treating schizophrenia, bipolar disorder, and stimulant psychosis. Several other dopamine antagonists are antiemetics used in the treatment of nausea and vomiting.

Dopaminergic means "related to dopamine", a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.

Amisulpride, sold under the brand names Solian and Barhemsys, is a medication used in the treatment of schizophrenia, acute psychotic episodes, depression, and nausea and vomiting. It is specifically used at lower doses intravenously to prevent and treat postoperative nausea and vomiting; at low doses by mouth to treat depression; and at higher doses by mouth to treat psychosis.

Sulpiride, sold under the brand name Dogmatil among others, is an atypical antipsychotic medication of the benzamide class which is used mainly in the treatment of psychosis associated with schizophrenia and major depressive disorder, and is sometimes used in low dosage to treat anxiety and mild depression.

Flupentixol (INN), also known as flupenthixol, marketed under brand names such as Depixol and Fluanxol is a typical antipsychotic drug of the thioxanthene class. It was introduced in 1965 by Lundbeck. In addition to single drug preparations, it is also available as flupentixol/melitracen—a combination product containing both melitracen and flupentixol . Flupentixol is not approved for use in the United States. It is, however, approved for use in the UK, Australia, Canada, Russian Federation, South Africa, New Zealand, Philippines, Iran, Germany, and various other countries.

Nemonapride, also previously known as emonapride and sold under the brand name Emilace, is an atypical antipsychotic which is used in the treatment of schizophrenia. It is taken by mouth.
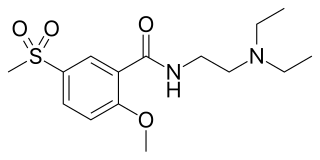
Tiapride is a drug that selectively blocks D2 and D3 dopamine receptors in the brain. It is used to treat a variety of neurological and psychiatric disorders including dyskinesia, alcohol withdrawal syndrome, negative symptoms of psychosis, and agitation and aggression in the elderly. A derivative of benzamide, tiapride is chemically and functionally similar to other benzamide antipsychotics such as sulpiride and amisulpride known for their dopamine antagonist effects.

Sultopride (trade names Barnetil, Barnotil, Topral) is an atypical antipsychotic of the benzamide chemical class used in Europe, Japan, and Hong Kong for the treatment of schizophrenia. It was launched by Sanofi-Aventis in 1976. Sultopride acts as a selective D2 and D3 receptor antagonist. It has also been shown to have clinically relevant affinity for the GHB receptor as well, a property it shares in common with amisulpride and sulpiride.

Perospirone (Lullan) is an atypical antipsychotic of the azapirone family. It was introduced in Japan by Dainippon Sumitomo Pharma in 2001 for the treatment of schizophrenia and acute cases of bipolar mania.

Mosapramine (Cremin) is an atypical antipsychotic used in Japan for the treatment of schizophrenia. It is a potent dopamine antagonist with high affinity to the D2, D3, and D4 receptors, and with moderate affinity for the 5-HT2 receptors.

UH-232 ((+)-UH232) is a drug which acts as a subtype selective mixed agonist-antagonist for dopamine receptors, acting as a weak partial agonist at the D3 subtype, and an antagonist at D2Sh autoreceptors on dopaminergic nerve terminals. It causes dopamine release in the brain and has a stimulant effect, as well as blocking the behavioural effects of cocaine. It may also serve as a 5-HT2A receptor agonist, based on animal studies. It was investigated in clinical trials for the treatment of schizophrenia, but unexpectedly caused symptoms to become worse.
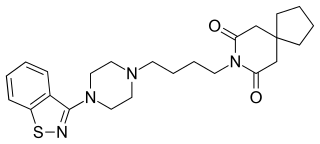
Tiospirone (BMY-13,859), also sometimes called tiaspirone or tiosperone, is an atypical antipsychotic of the azapirone class. It was investigated as a treatment for schizophrenia in the late 1980s and was found to have an effectiveness equivalent to those of typical antipsychotics in clinical trials but without causing extrapyramidal side effects. However, development was halted and it was not marketed. Perospirone, another azapirone derivative with antipsychotic properties, was synthesized and assayed several years after tiospirone. It was found to be both more potent and more selective in comparison and was commercialized instead.

N-Desmethylclozapine (NDMC), or norclozapine, is a major active metabolite of the atypical antipsychotic drug clozapine.
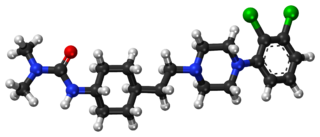
Cariprazine, sold under the brand name Vraylar among others, is an atypical antipsychotic developed by Gedeon Richter, which is used in the treatment of schizophrenia, bipolar mania, bipolar depression, and major depressive disorder. It acts primarily as a D3 and D2 receptor partial agonist, with a preference for the D3 receptor. Cariprazine is also a partial agonist at the serotonin 5-HT1A receptor and acts as an antagonist at 5-HT2B and 5-HT2A receptors, with high selectivity for the D3 receptor. It is taken by mouth.
Panamesine (INNTooltip International Nonproprietary Name; developmental code name EMD-57455) is a sigma receptor antagonist that was under development by Merck as a potential antipsychotic for the treatment of schizophrenia in the 1990s but was never marketed. It is a selective antagonist of both sigma receptor subtypes, the σ1 and σ2 receptors (IC50 = 6 nM). In addition, the major metabolite of the drug, EMD-59983, has high affinity for the sigma receptors (IC50 = 24 nM) and the dopamine D2 (IC50 = 23 nM) and D3 receptors, with potent antidopaminergic activity. Panamesine reached phase II clinical trials for schizophrenia prior to the discontinuation of its development.

SEP-4199, also known as non-racemic amisulpride, is a non-racemic form of amisulpride which is under development for the treatment of bipolar depression. It is taken by mouth.

The conditioned avoidance response (CAR) test, also known as the active avoidance test, is an animal test used to identify drugs with antipsychotic-like effects. It is most commonly employed as a two-way active avoidance test with rodents. The test assesses the conditioned ability of an animal to avoid an unpleasant stimulus. Drugs that selectively suppress conditioned avoidance responses without affecting escape behavior are considered to have antipsychotic-like activity. Variations of the test, like testing for enhancement of avoidance and escape responses, have also been used to assess other drug effects, like pro-motivational and antidepressant-like effects.

ENX-104, also known as deuterated nemonapride enantiomer, is a selective dopamine D2 and D3 receptor antagonist which is under development for the treatment of major depressive disorder. It is specifically under development for the treatment of major depressive disorder characterized by anhedonia. The drug is being developed for use at low doses to preferentially block presynaptic dopamine D2 and D3 autoreceptors and hence to enhance rather than inhibit dopaminergic neurotransmission. It is taken by mouth.


















