Dieting is the practice of eating food in a regulated way to decrease, maintain, or increase body weight, or to prevent and treat diseases such as diabetes and obesity. As weight loss depends on calorie intake, different kinds of calorie-reduced diets, such as those emphasising particular macronutrients, have been shown to be no more effective than one another. As weight regain is common, diet success is best predicted by long-term adherence. Regardless, the outcome of a diet can vary widely depending on the individual.

Low-carbohydrate diets restrict carbohydrate consumption relative to the average diet. Foods high in carbohydrates are limited, and replaced with foods containing a higher percentage of fat and protein, as well as low carbohydrate foods.
A low-protein diet is a diet in which people decrease their intake of protein. A low-protein diet is used as a therapy for inherited metabolic disorders, such as phenylketonuria and homocystinuria, and can also be used to treat kidney or liver disease. Low protein consumption appears to reduce the risk of bone breakage, presumably through changes in calcium homeostasis. Consequently, there is no uniform definition of what constitutes low-protein, because the amount and composition of protein for an individual with phenylketonuria would differ substantially from one with homocystinuria or tyrosinemia.

In nutrition, diet is the sum of food consumed by a person or other organism. The word diet often implies the use of specific intake of nutrition for health or weight-management reasons. Although humans are omnivores, each culture and each person holds some food preferences or some food taboos. This may be due to personal tastes or ethical reasons. Individual dietary choices may be more or less healthy.

Gastric bypass surgery refers to a technique in which the stomach is divided into a small upper pouch and a much larger lower "remnant" pouch and then the small intestine is rearranged to connect to both. Surgeons have developed several different ways to reconnect the intestine, thus leading to several different gastric bypass procedures (GBP). Any GBP leads to a marked reduction in the functional volume of the stomach, accompanied by an altered physiological and physical response to food.
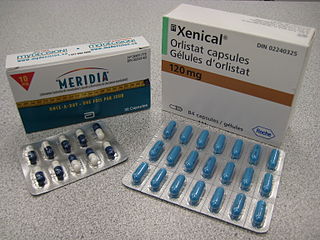
Anti-obesity medication or weight loss medications are pharmacological agents that reduce or control excess body fat. These medications alter one of the fundamental processes of the human body, weight regulation, by: reducing appetite and consequently energy intake, increasing energy expenditure, redirecting nutrients from adipose to lean tissue, or interfering with the absorption of calories.
Calorie restriction is a dietary regimen that reduces the energy intake from foods and beverages without incurring malnutrition. The possible effect of calorie restriction on body weight management, longevity, and aging-associated diseases has been an active area of research.

A high-protein diet is a diet in which 20% or more of the total daily calories come from protein. Many high protein diets are high in saturated fat and restrict intake of carbohydrates.

Protein–energy undernutrition (PEU), once called protein-energy malnutrition (PEM), is a form of malnutrition that is defined as a range of conditions arising from coincident lack of dietary protein and/or energy (calories) in varying proportions. The condition has mild, moderate, and severe degrees.

A very-low-calorie diet (VLCD), also known as semistarvation diet and crash diet, is a type of diet with very or extremely low daily food energy consumption. VLCDs are defined as a diet of 800 kilocalories (3,300 kJ) per day or less. Modern medically supervised VLCDs use total meal replacements, with regulated formulations in Europe and Canada which contain the recommended daily requirements for vitamins, minerals, trace elements, fatty acids, protein and electrolyte balance. Carbohydrates may be entirely absent, or substituted for a portion of the protein; this choice has important metabolic effects. Medically supervised VLCDs have specific therapeutic applications for rapid weight loss, such as in morbid obesity or before a bariatric surgery, using formulated, nutritionally complete liquid meals containing 800 kilocalories or less per day for a maximum of 12 weeks.

Enterostatin is a pentapeptide derived from a proenzyme in the gastrointestinal tract called procolipase. It reduces food intake, in particular fat intake, when given peripherally or into the brain.

Tesofensine (NS2330) is a serotonin–noradrenaline–dopamine reuptake inhibitor from the phenyltropane family of drugs, which is being developed for the treatment of obesity. Tesofensine was originally developed by a Danish biotechnology company, NeuroSearch, who transferred the rights to Saniona in 2014.

The central melanocortin system is defined anatomically as a collection of central nervous system circuits which include:
The diet-induced obesity model is an animal model used to study obesity using animals that have obesity caused by being fed high-fat or high-density diets. It is intended to mimic the most common cause of obesity in humans. Typically mice, rats, dogs, or non-human primates are used in these models. These animals can then be used to study in vivo obesity, obesity's comorbidities, and other related diseases. Users of such models must take into account the duration and type of diet as well as the environmental conditions and age of the animals, as each may promote different bodyweights, fat percentages, or behaviors.

Enobosarm, also formerly known as ostarine and by the developmental code names GTx-024, MK-2866, and S-22, is a selective androgen receptor modulator (SARM) which is under development for the treatment of androgen receptor-positive breast cancer in women and for improvement of body composition in people taking GLP-1 receptor agonists like semaglutide. It was also under development for a variety of other indications, including treatment of cachexia, Duchenne muscular dystrophy, muscle atrophy or sarcopenia, and stress urinary incontinence, but development for all other uses has been discontinued. Enobosarm was evaluated for the treatment of muscle wasting related to cancer in late-stage clinical trials, and the drug improved lean body mass in these trials, but it was not effective in improving muscle strength. As a result, enobosarm was not approved and development for this use was terminated. Enobosarm is taken by mouth.
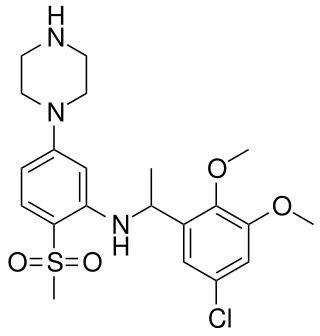
PRX-07034 is a selective 5-HT6 receptor antagonist. It has cognition and memory-enhancing properties and potently decreases food intake and body weight in rodents. PRX-07034 was under development by Epix Pharmaceuticals for the treatment of obesity and cognitive impairment associated with Alzheimer's disease and schizophrenia but upon the company collapsing due to lack of funds the compound was auctioned to another corporation.

Weight management refers to behaviors, techniques, and physiological processes that contribute to a person's ability to attain and maintain a healthy weight. Most weight management techniques encompass long-term lifestyle strategies that promote healthy eating and daily physical activity. Moreover, weight management involves developing meaningful ways to track weight over time and to identify the ideal body weights for different individuals.
Management of obesity can include lifestyle changes, medications, or surgery. Although many studies have sought effective interventions, there is currently no evidence-based, well-defined, and efficient intervention to prevent obesity.
George A. Bray is an American obesity researcher. As of 2016, he is a University Professor emeritus and formerly the chief of the division of clinical obesity and metabolism at Louisiana State University's Pennington Biomedical Research Center in Baton Rouge. He is also a Boyd Professor emeritus at the Pennington Center, and a professor of medicine emeritus at the Louisiana State University Medical Center.

Vosilasarm, also known by the development codes RAD140 and EP0062 and by the black-market name Testolone or Testalone, is a selective androgen receptor modulator (SARM) which is under development for the treatment of hormone-sensitive breast cancer. It is specifically under development for the treatment of androgen receptor-positive, estrogen receptor-negative, HER2-negative advanced breast cancer. Vosilasarm was also previously under development for the treatment of sarcopenia, osteoporosis, and weight loss due to cancer cachexia, but development for these indications was discontinued. The drug is taken by mouth.














