Ayahuasca is a South American psychoactive beverage, traditionally used by Indigenous cultures and folk healers in the Amazon and Orinoco basins for spiritual ceremonies, divination, and healing a variety of psychosomatic complaints.

N,N-Dimethyltryptamine is a substituted tryptamine that occurs in many plants and animals, including humans, and which is both a derivative and a structural analog of tryptamine. DMT is used as a psychedelic drug and prepared by various cultures for ritual purposes as an entheogen.

Banisteriopsis caapi, also known as, caapi, soul vine, yagé (yage), or ayahuasca, the latter of which also refers to the psychedelic decoction made with the vine and a plant source of dimethyltryptamine, is a South American liana of the family Malpighiaceae. It is commonly used as an ingredient of ayahuasca, a decoction with a long history of its entheogenic use and holds status as a "plant teacher" among the Indigenous peoples of the Amazon rainforest.

Tryptamine is an indolamine metabolite of the essential amino acid tryptophan. The chemical structure is defined by an indole—a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon. The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others.

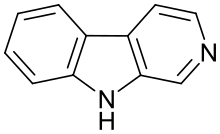
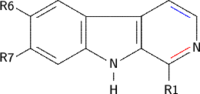
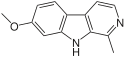
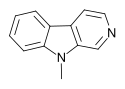
Harmala alkaloids are several alkaloids that act as monoamine oxidase inhibitors (MAOIs). These alkaloids are found in the seeds of Peganum harmala, as well as Banisteriopsis caapi (ayahuasca), leaves of tobacco and coffee beans. The alkaloids include harmine, harmaline, harmalol, and their derivatives, which have similar chemical structures, hence the name "harmala alkaloids". These alkaloids are of interest for their use in Amazonian shamanism, where they are derived from other plants. Harmine, once known as telepathine and banisterine, is a naturally occurring beta-carboline alkaloid that is structurally related to harmaline, and also found in the vine Banisteriopsis caapi. Tetrahydroharmine is also found in B. caapi and P. harmala. Dr. Alexander Shulgin has suggested that harmine may be a breakdown product of harmaline. Harmine and harmaline are reversible inhibitors of monoamine oxidase A (RIMAs). They can stimulate the central nervous system by inhibiting the metabolism of monoamine compounds such as serotonin and norepinephrine.

Peganum harmala, commonly called wild rue, Syrian rue, African rue, esfand or espand, or harmel, is a perennial, herbaceous plant, with a woody underground rootstock, of the family Nitrariaceae, usually growing in saline soils in temperate desert and Mediterranean regions. Its common English-language name came about because of a resemblance to rue. Because eating it would sicken or kill livestock, it is considered a noxious weed in a number of countries. It has become an invasive species in some regions of the western United States. The plant is popular in Middle Eastern and north African folk medicine. The alkaloids contained in the plant, including the seeds, are monoamine oxidase inhibitors.
Harmine is a beta-carboline and a harmala alkaloid. It occurs in a number of different plants, most notably the Syrian rue and Banisteriopsis caapi. Harmine reversibly inhibits monoamine oxidase A (MAO-A), an enzyme which breaks down monoamines, making it a Reversible inhibitor of monoamine oxidase A (RIMA). Harmine does not inhibit MAO-B. Harmine is also known as banisterin, banisterine, telopathin, telepathine, leucoharmine and yagin, yageine.

Harmaline is a fluorescent indole alkaloid from the group of harmala alkaloids and beta-carbolines. It is the partly hydrogenated form of harmine.

Tetrahydroharmine (THH) is a fluorescent indole alkaloid that occurs in the tropical liana species Banisteriopsis caapi.
Pharmahuasca is a pharmaceutical version of the entheogenic brew ayahuasca. Traditional ayahuasca is made by brewing the MAOI-containing Banisteriopsis caapi vine with a DMT-containing plant, such as Psychotria viridis. Pharmahuasca refers to a similar combination that uses a pharmaceutical MAOI instead of a plant.

Dopaminergic means "related to dopamine", a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Tryptoline, also known as tetrahydro-β-carboline and tetrahydronorharmane, is a natural organic derivative of beta-carboline. It is an alkaloid chemically related to tryptamines. Derivatives of tryptoline have a variety of pharmacological properties and are known collectively as tryptolines.

Tetrahydroharman(e), also known as 1-methyl-1,2,3,4-tetrahydro-β-carboline, is a general name for one of two isomers:
- (1S)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole
- Calligonine ((1R)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole)

Harmane (harman) is a heterocyclic amine found in a variety of foods including coffee, sauces, and cooked meat. It is also present in tobacco smoke.

Eudistomins are β-carboline derivatives, isolated from ascidians, like Ritterella sigillinoides, Lissoclinum fragile, or Pseudodistoma aureum.
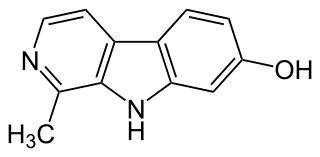
Harmol is a chemical compound classified as a β-carboline. It is readily formed in vivo in humans by O-demethylation of harmine.

9-Methyl-β-carboline (9-Me-BC) is a heterocyclic amine of the β-carboline family, and a research chemical.
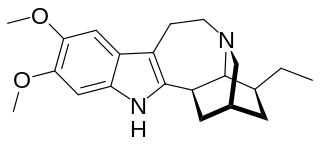
Ibogaline is an alkaloid found in Tabernanthe iboga along with the related chemical compounds ibogaine, ibogamine, and other minor alkaloids. It is a relatively smaller component of Tabernanthe iboga root bark total alkaloids (TA) content. It is also present in Tabernaemontana species such as Tabernaemontana australis which shares similar ibogan-biosynthetic pathways. The percentage of ibogaline in T. iboga root bark is up to 15% TA with ibogaine constituting 80% of the alkaloids and ibogamine up to 5%.