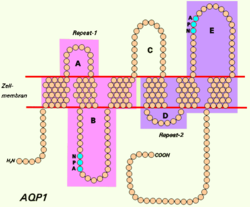
Aquaporin 1 (AQP-1) is a protein that in humans is encoded by the AQP1 gene.
Contents
AQP-1 is a widely expressed water channel, whose physiological function has been most thoroughly characterized in the kidney. It is found in the basolateral and apical plasma membranes of the proximal tubules, the descending limb of the loop of Henle, and in the descending portion of the vasa recta. Additionally, it is found in red blood cells, vascular endothelium, the gastrointestinal tract, sweat glands, lungs, and the central nervous system. [5]
Neural AQP-1 are regulated by vasopressin AVPR1A receptor activity. [6]