
Cathine, also known as D-norpseudoephedrine or as (+)-norpseudoephedrine, is a psychoactive drug of the phenethylamine and amphetamine groups which acts as a stimulant. Along with cathinone, it is found naturally in Catha edulis (khat), and contributes to the overall effects of the plant. Cathine has approximately 7 to 10% of the potency of amphetamine.

Cathinone is a monoamine alkaloid found in the shrub Catha edulis (khat) and is chemically similar to ephedrine, cathine, methcathinone and other amphetamines. It is probably the main contributor to the stimulant effect of Catha edulis, also known as khat. Cathinone differs from many other amphetamines in that it has a ketone functional group. Other phenethylamines that share this structure include the stimulants methcathinone, MDPV, mephedrone and the antidepressant bupropion.
Phentermine, sold under the brand name Adipex-P among others, is a medication used together with diet and exercise to treat obesity. It is available by itself or as the combination phentermine/topiramate. Phentermine is taken by mouth.

Chlorphentermine, sold under the brand names Apsedon, Desopimon, and Lucofen, is a serotonergic appetite suppressant of the amphetamine family. Developed in 1962, it is the para-chloro derivative of the better-known appetite suppressant phentermine, which is still in current use.

Etilamfetamine, also known as N-ethylamphetamine and formerly sold under the brand names Apetinil and Adiparthrol, is a stimulant drug of the amphetamine family. It was invented in the early 20th century and was subsequently used as an anorectic or appetite suppressant in the 1950s, but was not as commonly used as other amphetamines such as amphetamine, methamphetamine, and benzphetamine, and was largely discontinued once newer drugs such as phenmetrazine were introduced.

Naphthylaminopropane, also known as naphthylisopropylamine (NIPA), is an experimental drug that was under investigation for the treatment of alcohol and stimulant addiction.

Norfenfluramine, or 3-trifluoromethylamphetamine, is a never-marketed drug of the amphetamine family and a major active metabolite of the appetite suppressants fenfluramine and benfluorex. The compound is a racemic mixture of two enantiomers with differing activities, dexnorfenfluramine and levonorfenfluramine.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; MRAs can induce the release of one or more of these neurotransmitters.

A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain.

A norepinephrine–dopamine releasing agent (NDRA) is a type of drug which induces the release of norepinephrine and dopamine in the body and/or brain.
A serotonin–dopamine releasing agent (SDRA) is a type of drug which induces the release of serotonin and dopamine in the body and/or brain.

4-Methylmethamphetamine (4-MMA), also known as mephedrine, is a putative stimulant and entactogen drug of the amphetamine family. It acts as a serotonin–norepinephrine–dopamine releasing agent (SNDRA). The drug is the β-deketo analogue of mephedrone and the N-methyl analogue of 4-methylamphetamine (4-MA).

Substituted cathinones, or simply cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

3-Methylmethcathinone (3-MMC), also known as metaphedrone, is a designer drug from the substituted cathinone family. 3-MMC is a monoamine transporter substrate that potently releases and inhibits the reuptake of dopamine and norepinephrine, as well as displaying moderate serotonin releasing activity.

3-Chloromethcathinone (3-CMC), also known as clophedrone, is a synthetic substance belonging to the cathinone class of psychoactive compounds. It is very similar in structure to other methcathinone derivatives such as 3-MMC and 4-CMC. Unlike cathinone, which occurs naturally in the khat plant Catha edulis, 3-CMC is not found in nature and is solely produced through chemical synthesis.
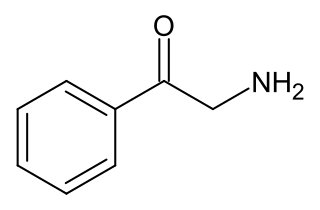
2-Aminoacetophenone, also known as β-ketophenethylamine, α-desmethylcathinone, or phenacylamine, is a substituted phenethylamine derivative. It is the phenethylamine homologue of cathinone (β-ketoamphetamine) and hence is a parent compound of a large number of stimulant and entactogen drugs.

BK-NM-AMT, or βk-NM-αMT, also known as β-keto-N-methyl-αMT or as α,N-dimethyl-β-ketotryptamine, is a serotonin–dopamine releasing agent (SDRA) and putative entactogen of the tryptamine and α-alkyltryptamine families. Along with certain other tryptamines, such as α-ethyltryptamine (αET), 5-chloro-αMT and 5-fluoro-αET, it is one of the few SDRAs known.

2-Fluoromethcathinone (2-FMC), also known as 2-flephedrone, is a psychostimulant and designer drug of the cathinone family. It acts as a dopamine and norepinephrine releasing agent (NDRA).

2-Phenylmorpholine is the parent compound of the substituted phenylmorpholine class of compounds. Examples of 2-phenylmorpholine derivatives include phenmetrazine (3-methyl-2-phenylmorpholine), phendimetrazine ( -3,4-dimethyl-2-phenylmorpholine), and pseudophenmetrazine ( -3-methyl-2-phenylmorpholine), which are monoamine releasing agents (MRAs) and psychostimulants. 2-Phenylmorpholine itself is a potent norepinephrine–dopamine releasing agent (NDRA) and hence may act as a stimulant similarly.
















