Hyperbaric medicine is a medical treatment in which an increase in barometric pressure over ambient pressure is employed increasing the partial pressures of all gases present in the compressed air. The immediate effects include reducing the size of gas embolisms and raising the partial pressures of all gases present according to Henry's law. Currently, there are two types of hyperbaric medicine depending on the gases compressed, hyperbaric air and hyperbaric oxygen.

A diving regulator or underwater diving regulator is a pressure regulator that controls the pressure of breathing gas for underwater diving. The most commonly recognised application is to reduce pressurized breathing gas to ambient pressure and deliver it to the diver, but there are also other types of gas pressure regulator used for diving applications. The gas may be air or one of a variety of specially blended breathing gases. The gas may be supplied from a scuba cylinder carried by the diver, in which case it is called a scuba regulator, or via a hose from a compressor or high-pressure storage cylinders at the surface in surface-supplied diving. A gas pressure regulator has one or more valves in series which reduce pressure from the source, and use the downstream pressure as feedback to control the delivered pressure, or the upstream pressure as feedback to prevent excessive flow rates, lowering the pressure at each stage.

Saturation diving is diving for periods long enough to bring all tissues into equilibrium with the partial pressures of the inert components of the breathing gas used. It is a diving mode that reduces the number of decompressions divers working at great depths must undergo by only decompressing divers once at the end of the diving operation, which may last days to weeks, having them remain under pressure for the whole period. A diver breathing pressurized gas accumulates dissolved inert gas used in the breathing mixture to dilute the oxygen to a non-toxic level in the tissues, which can cause potentially fatal decompression sickness if permitted to come out of solution within the body tissues; hence, returning to the surface safely requires lengthy decompression so that the inert gases can be eliminated via the lungs. Once the dissolved gases in a diver's tissues reach the saturation point, however, decompression time does not increase with further exposure, as no more inert gas is accumulated.
In-water recompression (IWR) or underwater oxygen treatment is the emergency treatment of decompression sickness (DCS) by returning the diver underwater to help the gas bubbles in the tissues, which are causing the symptoms, to resolve. It is a procedure that exposes the diver to significant risk which should be compared with the risk associated with the available options and balanced against the probable benefits. Some authorities recommend that it is only to be used when the time to travel to the nearest recompression chamber is too long to save the victim's life; others take a more pragmatic approach and accept that in some circumstances IWR is the best available option. The risks may not be justified for case of mild symptoms likely to resolve spontaneously, or for cases where the diver is likely to be unsafe in the water, but in-water recompression may be justified in cases where severe outcomes are likely if not recompressed, if conducted by a competent and suitably equipped team.

A breathing apparatus or breathing set is equipment which allows a person to breathe in a hostile environment where breathing would otherwise be impossible, difficult, harmful, or hazardous, or assists a person to breathe. A respirator, medical ventilator, or resuscitator may also be considered to be breathing apparatus. Equipment that supplies or recycles breathing gas other than ambient air in a space used by several people is usually referred to as being part of a life-support system, and a life-support system for one person may include breathing apparatus, when the breathing gas is specifically supplied to the user rather than to the enclosure in which the user is the occupant.

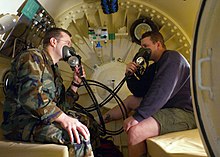
An oxygen mask is a mask that provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth or the entire face. They may be made of plastic, silicone, or rubber. In certain circumstances, oxygen may be delivered via a nasal cannula instead of a mask.

A diving chamber is a vessel for human occupation, which may have an entrance that can be sealed to hold an internal pressure significantly higher than ambient pressure, a pressurised gas system to control the internal pressure, and a supply of breathing gas for the occupants.

Diver rescue, usually following an accident, is the process of avoiding or limiting further exposure to diving hazards and bringing a diver to a place of safety. A safe place generally means a place where the diver cannot drown, such as a boat or dry land, where first aid can be administered and from which professional medical treatment can be sought. In the context of surface supplied diving, the place of safety for a diver with a decompression obligation is often the diving bell.

The breathing performance of regulators is a measure of the ability of a breathing gas regulator to meet the demands placed on it at varying ambient pressures and temperatures, and under varying breathing loads, for the range of breathing gases it may be expected to deliver. Performance is an important factor in design and selection of breathing regulators for any application, but particularly for underwater diving, as the range of ambient operating pressures and temperatures, and variety of breathing gases is broader in this application. A diving regulator is a device that reduces the high pressure in a diving cylinder or surface supply hose to the same pressure as the diver's surroundings. It is desirable that breathing from a regulator requires low effort even when supplying large amounts of breathing gas as this is commonly the limiting factor for underwater exertion, and can be critical during diving emergencies. It is also preferable that the gas is delivered smoothly without any sudden changes in resistance while inhaling or exhaling, and that the regulator does not lock up and either fail to supply gas or free-flow. Although these factors may be judged subjectively, it is convenient to have standards by which the many different types and manufactures of regulators may be objectively compared.

An orinasal mask, oro-nasal mask or oral-nasal mask is a breathing mask that covers the mouth and the nose only. It may be a complete independent item, as an oxygen mask, or on some anaesthetic apparatuses, or it may be fitted as a component inside a fullface mask on underwater breathing apparatus, a gas mask or an industrial respirator to reduce the amount of dead space. It may be designed for its lower edge to seal on the front of the lower jaw or to go under the chin.

To prevent or minimize decompression sickness, divers must properly plan and monitor decompression. Divers follow a decompression model to safely allow the release of excess inert gases dissolved in their body tissues, which accommodated as a result of breathing at ambient pressures greater than surface atmospheric pressure. Decompression models take into account variables such as depth and time of dive, breathing gasses, altitude, and equipment to develop appropriate procedures for safe ascent.

Hyperbaric treatment schedules or hyperbaric treatment tables, are planned sequences of events in chronological order for hyperbaric pressure exposures specifying the pressure profile over time and the breathing gas to be used during specified periods, for medical treatment. Hyperbaric therapy is based on exposure to pressures greater than normal atmospheric pressure, and in many cases the use of breathing gases with oxygen content greater than that of air.

There are several categories of decompression equipment used to help divers decompress, which is the process required to allow divers to return to the surface safely after spending time underwater at higher ambient pressures.

The metresea water (msw) is a metric unit of pressure used in underwater diving. It is defined as one tenth of a bar.

The following index is provided as an overview of and topical guide to underwater diving:
Demand Valve Oxygen Therapy (DVOT) is a way of delivering high flow oxygen therapy using a device that only delivers oxygen when the patient breathes in and shuts off when they breathe out. DVOT is commonly used to treat conditions such as cluster headache, which affects up to four in 1000 people (0.4%), and is a recommended first aid procedure for several diving disorders. It is also a recommended prophylactic for decompression sickness in the event of minor omitted decompression without symptoms.
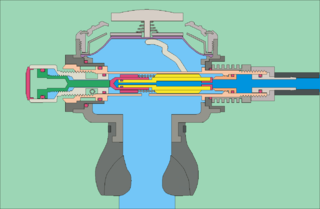
The mechanism of diving regulators is the arrangement of components and function of gas pressure regulators used in the systems which supply breathing gases for underwater diving. Both free-flow and demand regulators use mechanical feedback of the downstream pressure to control the opening of a valve which controls gas flow from the upstream, high-pressure side, to the downstream, low-pressure side of each stage. Flow capacity must be sufficient to allow the downstream pressure to be maintained at maximum demand, and sensitivity must be appropriate to deliver maximum required flow rate with a small variation in downstream pressure, and for a large variation in supply pressure, without instability of flow. Open circuit scuba regulators must also deliver against a variable ambient pressure. They must be robust and reliable, as they are life-support equipment which must function in the relatively hostile seawater environment, and the human interface must be comfortable over periods of several hours.
The US Navy has used several decompression models from which their published decompression tables and authorized diving computer algorithms have been derived. The original C&R tables used a classic multiple independent parallel compartment model based on the work of J.S.Haldane in England in the early 20th century, using a critical ratio exponential ingassing and outgassing model. Later they were modified by O.D. Yarborough and published in 1937. A version developed by Des Granges was published in 1956. Further developments by M.W. Goodman and Robert D. Workman using a critical supersaturation approach to incorporate M-values, and expressed as an algorithm suitable for programming were published in 1965, and later again a significantly different model, the VVAL 18 exponential/linear model was developed by Edward D. Thalmann, using an exponential ingassing model and a combined exponential and linear outgassing model, which was further developed by Gerth and Doolette and published in Revision 6 of the US Navy Diving Manual as the 2008 tables.

The following index is provided as an overview of and topical guide to underwater diving: