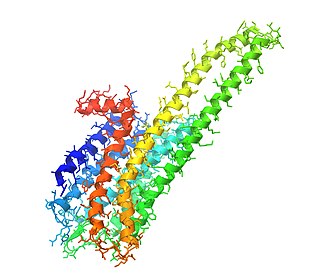
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

meta-Chlorophenylpiperazine (mCPP) is a psychoactive drug of the phenylpiperazine class. It was initially developed in the late-1970s and used in scientific research before being sold as a designer drug in the mid-2000s. It has been detected in pills touted as legal alternatives to illicit stimulants in New Zealand and pills sold as "ecstasy" in Europe and the United States.
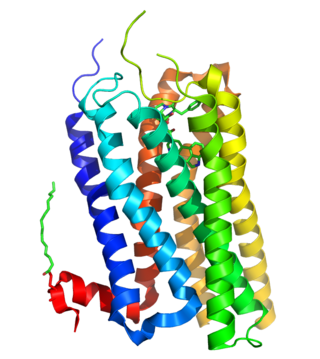
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, the 5-HT2B receptor is Gq/G11-protein coupled, leading to downstream activation of phospholipase C.

Xylamidine is a drug which acts as an antagonist of the serotonin 5-HT2A and 5-HT2C receptors, and to a lesser extent of the serotonin 5-HT1A receptor. The drug does not cross the blood–brain barrier and hence is peripherally selective, which makes it useful for blocking peripheral serotonergic responses like cardiovascular and gastrointestinal effects, without producing the central effects of 5-HT2A receptor blockade such as sedation, or interfering with the central actions of 5-HT2A receptor agonists.

YM-348 is an indazole derivative drug which acts as a potent and selective 5-HT2C receptor agonist, with an EC50 of 1nM and 15x selectivity over 5-HT2A, although it only has moderate selectivity of 3x over the closely related 5-HT2B receptor. It has thermogenic and anorectic effects in animal studies, making it potentially useful for the treatment of obesity.

Ro60-0175 is a drug of the isotryptamine group developed by Hoffmann–La Roche, which has applications in scientific research. It acts as a potent and selective agonist for both the 5-HT2B and 5-HT2C serotonin receptor subtypes, with good selectivity over the closely related 5-HT2A subtype, and little or no affinity at other receptors.

PNU-22394 is a drug which acts as an agonist at serotonin 5-HT2 receptors, with strongest binding affinity for 5-HT2A and 5-HT2C and slightly weaker at 5-HT2B, although it is only a full agonist at 5-HT2C, but partial agonist at 5-HT2A and 5-HT2B. It has anorectic effects in both animal studies and human trials, along with "Pro-Cognitive Properties", although it has never been developed for medical use.
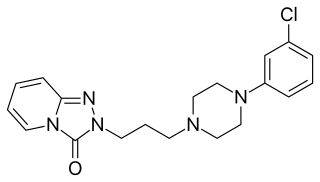
Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.

4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring. They are 4-substituted derivatives of 2,5-dimethoxyamphetamine.

VER-3323 is a drug which acts as a selective agonist for both the 5-HT2B and 5-HT2C serotonin receptor subtypes, with moderate selectivity for 5-HT2C, but relatively low affinity for 5-HT2A. It has potent anorectic effects in animal studies.

PRX-08066 is a drug discovered and developed by Predix Pharmaceuticals [Dale S. Dhanoa et al. Patent US 7,030,240 B2], which acts as a potent and selective antagonist at the serotonin 5-HT2B receptor, with a 5-HT2Bbinding affinity (Ki) of 3.4nM, and high selectivity over the closely related 5-HT2A and 5-HT2C receptors and other receptor targets. PRX-08066 and other selective 5-HT2B antagonists are being researched for the treatment of pulmonary arterial hypertension, following the discovery that the potent 5-HT2B agonist norfenfluramine produces pulmonary arterial hypertension and subsequent heart valve damage. In animal studies, PRX-08066 has been found to reduce several key indicators of pulmonary arterial hypertension and improved cardiac output, with similar efficacy to established drugs for this condition such as bosentan, sildenafil, beraprost and iloprost. It is also being researched for potential anti-cancer applications, due to its ability to inhibit fibroblast activation.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors.

5-MeO-NBpBrT is a N-substituted member of the methoxytryptamine family of compounds. Like other such compounds it acts as an antagonist for the 5-HT2A receptor, with a claimed 100x selectivity over the closely related 5-HT2C receptor. While N-benzyl substitution of psychedelic phenethylamines often results in potent 5-HT2A agonists, it had been thought that N-benzyl tryptamines show much lower efficacy and are either very weak partial agonists or antagonists at 5-HT2A, though more recent research has shown stronger agonist activity for 3-substituted benzyl derivatives. Extending the benzyl group to a substituted phenethyl can also recover agonist activity in certain cases.

WAY-161503 is a full agonist of 5-HT2C receptors (Ki = 3.3 nM for displacement of DOI), ~6-fold less potent at 5-HT2A receptors (Ki = 18 nM) and 20-fold less potent at 5-HT2B receptors (Ki = 60 nM). In functional studies, it stimulates calcium mobilization coupled to 5-HT2C, 5-HT2B, and 5-HT2A receptors with EC50 values of 0.8, 1.8, and 7 nM, respectively. WAY-161503 has been reported to produce dose-dependent decreases in food intake in 24-hour fasted normal Sprague-Dawley rats, diet-induced obese mice, and obese Zucker rats with ED50 values of 1.9, 6.8, and 0.73 mg/kg, respectively.
5-HT2C receptor agonists are a class of drugs that activate 5-HT2C receptors. They have been investigated for the treatment of a number of conditions including obesity, psychiatric disorders, sexual dysfunction and urinary incontinence.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. It is a member of the NBOMe family of psychedelics.

25N-NBOMe is a derivative of the hallucinogen 2C-N. The pharmacological properties of 25N-NBOMe have not been described in the scientific literature, but it is believed to act in a similar manner to related compounds such as 25I-NBOMe and 25C-NBOMe, which are potent agonists at the 5HT2A receptor. 25N-NBOMe has been sold as a street drug and has only been described in the literature in terms of identification by forensic analysis.

1-Methylpsilocin (developmental code names CMY, CMY-16) is a tryptamine derivative developed by Sandoz which acts as a selective agonist of the serotonin 5-HT2C receptor (IC50Tooltip half-maximal inhibitory concentration of 12 nM, vs. 633 nM at 5-HT2A), and an inverse agonist at 5-HT2B (Ki of 38 nM). While 1-methylpsilocin does have higher affinity for 5-HT2C than 5-HT2A, it does produce a head-twitch response in mice that is dependent on 5-HT2A, so it is not entirely free of effects on 5-HT2Ain vivo. In contrast to psilocin, 1-methylpsilocin did not activate 5-HT1A receptors in mice.

The 25-NB (25x-NBx) series, or NBOMe series, also known as the N-benzylphenethylamines, is a family of serotonergic psychedelics. They are substituted phenethylamines and were derived from the 2C family. The most commonly encountered NBOMe drugs are 25I-NBOMe, 25B-NBOMe, and 25C-NBOMe.

SB-221284 is a selective serotonin 5-HT2C and 5-HT2B receptor antagonist which is used in scientific research.



















