This article needs additional citations for verification .(November 2023) |
 | |
| Names | |
|---|---|
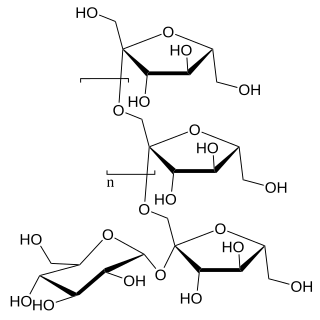
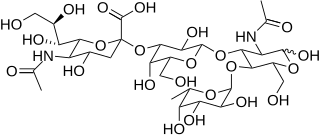
| IUPAC name (5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-[α-L-fucopyranosyl-(1→3)]-N-acetyl-D-glucosamine | |
| Systematic IUPAC name (2S,4S,5R,6R)-5-Acetamido-2-{[(2S,3R,4S,5S,6R)-2-{[(2R,3R,4R,5R)-5-acetamido-1,2-dihydroxy-6-oxo-3-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}hexan-3-yl]oxy}-2,4-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid | |
| Other names sialyl LeX, SLeX, CD15s, SSEA-1 | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| MeSH | sialyl+Lewis+X |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C31H52N2O23 | |
| Molar mass | 820.744 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
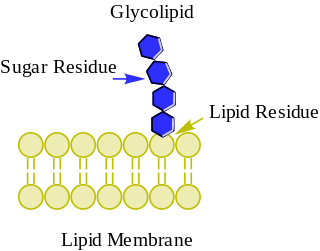
Sialyl LewisX (sLeX), also known as cluster of differentiation 15s (CD15s) or stage-specific embryonic antigen 1 (SSEA-1), is a tetrasaccharide carbohydrate which is usually attached to O-glycans on the surface of cells. It is known to play a vital role in cell-to-cell recognition processes. It is also the means by which an egg attracts sperm; first, to stick to it, then bond with it and eventually form a zygote.
Contents
- Structure
- Function
- Leukocyte homing
- Fertilization
- Clinical significance
- Leukocyte adhesion deficiency
- Blood cancers
- Cancer metastasis
- In vitro fertilization
- Immunity and inflammation
- MERS coronavirus binding
- History
- See also
- References
- Further reading
Sialyl-LewisX is also one of the most important blood group antigens and is displayed on the terminus of glycolipids that are present on the cell surface. The sialyl-LewisX determinant, E-selectin ligand carbohydrate structure, is constitutively expressed on granulocytes and monocytes and mediates inflammatory extravasation of these cells. Resting T and B lymphocytes lack its expression and are induced to strongly express sialyl-LewisX upon activation. The sialyl-LewisX determinant is expressed preferentially on activated Th1 cells but not on Th2 cells.