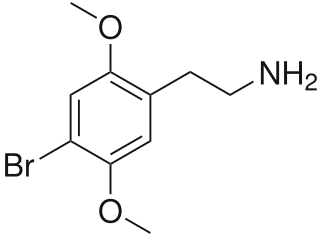
2C-B (4-bromo-2,5-dimethoxyphenethylamine), also known as Nexus, is a synthetic psychedelic drug of the 2C family, mainly used as a recreational drug. It was first synthesized by Alexander Shulgin in 1974 for use in psychotherapy. To date, there is limited scientific information regarding the drug's pharmacokinetics and pharmacological effects in humans. The existing studies primarily classify 2C-B as a stimulant and hallucinogen, and less commonly an entactogen and empathogen.

2,5-Dimethoxy-4-methylamphetamine is a psychedelic and a substituted amphetamine. It was first synthesized by Alexander Shulgin, and later reported in his book PiHKAL: A Chemical Love Story. DOM is classified as a Schedule I substance in the United States, and is similarly controlled in other parts of the world. Internationally, it is a Schedule I drug under the Convention on Psychotropic Substances. It is generally taken orally.

Dimethoxybromoamphetamine (DOB), also known as brolamfetamine and bromo-DMA, is a psychedelic drug and substituted amphetamine of the phenethylamine class of compounds. DOB was first synthesized by Alexander Shulgin in 1967. Its synthesis and effects are documented in Shulgin's book PiHKAL: A Chemical Love Story.

2C-T-4 (2,5-dimethoxy-4-isopropylthiophenethylamine) is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin and is used as entheogenic recreational drug.

2,4,5-Trimethoxyamphetamine (2,4,5-TMA), also known as TMA-2 or as 2,5-dimethoxy-4-methoxyamphetamine (DOMeO), is a psychedelic drug of the phenethylamine and amphetamine families. It is one of the trimethoxyamphetamine (TMA) series of positional isomers. The drug is also notable in being the 4-methoxylated member of the DOx series of drugs.
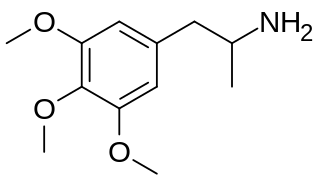
Trimethoxyamphetamine, also known as 3,4,5-trimethoxyamphetamine (3,4,5-TMA), α-methylmescaline, or mescalamphetamine, is a psychedelic drug of the phenethylamine and amphetamine families. It is one of the trimethoxyamphetamine (TMA) series of positional isomers. The drug is notable in being the amphetamine analogue of mescaline (3,4,5-trimethoxyphenethylamine).

2C-T is a psychedelic and hallucinogenic drug of the 2C family. It is used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs mescaline and 2C-T-2.

2C-TFM is a psychedelic phenethylamine of the 2C family. It was first synthesized in the laboratory of David E. Nichols. It has also been called 2C-CF3, a name derived from the Para-trifluoromethyl group it contains.

2,5-Dimethoxy-4-ethylamphetamine (DOET) is a psychedelic drug of the phenethylamine, amphetamine, and DOx families. It is closely related to DOM and is a synthetic analogue of the naturally occurring phenethylamine psychedelic mescaline. The drug acts as a selective agonist of the serotonin 5-HT2 receptors, including of the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors.
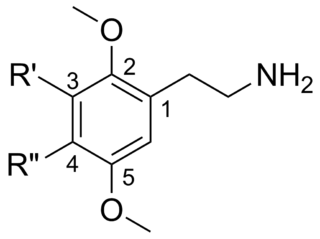
2C (2C-x) is a general name for the family of psychedelic phenethylamines containing methoxy groups on the 2 and 5 positions of a benzene ring. Most of these compounds also carry lipophilic substituents at the 4 position, usually resulting in more potent and more metabolically stable and longer acting compounds.

Aleph is a psychedelic hallucinogenic drug and a substituted amphetamine of the phenethylamine class of compounds, which can be used as an entheogen. It was first synthesized by Alexander Shulgin, who named it after the first letter of the Hebrew alphabet. In his book PiHKAL, Shulgin lists the dosage range as 5–10 mg, with effects typically lasting for 6 to 8 hours.

2C-T-13 is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL.

2C-T-15 or 2,5-dimethoxy-4-(β-cyclopropylthio)phenethylamine is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL .
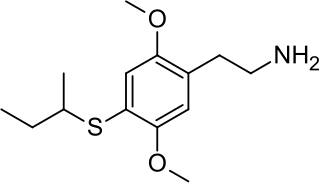
2C-T-17 or 2,5-dimethoxy-4-(β-secbutylthio)phenethylamine is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL .

2,5-Dimethoxy-4-butylamphetamine (DOBU) is a lesser-known psychedelic drug and a substituted amphetamine. DOBU was first synthesized by Alexander Shulgin. In his book PiHKAL , only low dosages of 2 to 3 mg were tested, with the duration simply listed as "very long". DOBU produces paresthesia and difficulty sleeping, but with few other effects.

Dimethoxy-4-amylamphetamine (DOAM) is a lesser-known psychedelic drug and a substituted amphetamine. DOAM was first synthesized by Alexander Shulgin. In his book PiHKAL , the minimum dosage is listed as 10 mg, and the duration is unknown. DOAM produces a bare threshold and tenseness.

2,5-Dimethoxyamphetamine (2,5-DMA), also known as DMA-4 or as DOH, is a drug of the phenethylamine and amphetamine families. It is one of the dimethoxyamphetamine (DMA) series of positional isomers. The drug is notable in being the parent compound of the DOx (4-substituted-2,5-dimethoxyamphetamine) series of drugs.

2,5-Dimethoxy-4-fluoroamphetamine (DOF) is a psychedelic drug of the phenethylamine and amphetamine classes. Alexander Shulgin briefly describes DOF in his book PiHKAL:
Animal studies that have compared DOF to the highly potent DOI and DOB imply that the human activity will be some four to six times less than these two heavier halide analogues.

4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring. They are 4-substituted derivatives of 2,5-dimethoxyamphetamine.

4C-B is a lesser-known psychedelic drug which is related to 2C-B and DOB. It is a reasonably potent 5-HT2A receptor partial agonist with a Ki of 7.6nM, but has relatively low efficacy. It is briefly mentioned in Alexander Shulgin's book PiHKAL but was never tested by him, however it has subsequently been tested by other researchers and was found to be active in a dose range of 50-80mg with a duration of around 8 hours, though with generally milder effects than 2C-B or DOB.



















