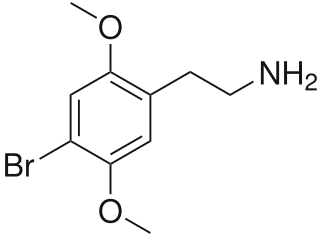
2C-B (4-bromo-2,5-dimethoxyphenethylamine), also known as Nexus, is a synthetic psychedelic drug of the 2C family, mainly used as a recreational drug. It was first synthesized by Alexander Shulgin in 1974 for use in psychotherapy. To date, there is limited scientific information regarding the drug's pharmacokinetics and pharmacological effects in humans. The existing studies primarily classify 2C-B as a stimulant and hallucinogen, and less commonly an entactogen and empathogen.

2,5-Dimethoxy-4-methylamphetamine is a psychedelic and a substituted amphetamine. It was first synthesized by Alexander Shulgin, and later reported in his book PiHKAL: A Chemical Love Story. DOM is classified as a Schedule I substance in the United States, and is similarly controlled in other parts of the world. Internationally, it is a Schedule I drug under the Convention on Psychotropic Substances. It is generally taken orally.

Dimethoxybromoamphetamine (DOB), also known as brolamfetamine and bromo-DMA, is a psychedelic drug and substituted amphetamine of the phenethylamine class of compounds. DOB was first synthesized by Alexander Shulgin in 1967. Its synthesis and effects are documented in Shulgin's book PiHKAL: A Chemical Love Story.

2C-T-8 is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin, sometimes used as an entheogen.

2C-T-4 (2,5-dimethoxy-4-isopropylthiophenethylamine) is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin and is used as entheogenic recreational drug.
MBDB, also known as N-methyl-1,3-benzodioxolylbutanamine or as 3,4-methylenedioxy-N-methyl-α-ethylphenylethylamine, is an entactogen of the phenethylamine, amphetamine, and phenylisobutylamine families related to MDMA. It is known by the street names "Eden" and "Methyl-J".

2C-T is a psychedelic and hallucinogenic drug of the 2C family. It is used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs mescaline and 2C-T-2.

2C-TFM is a psychedelic phenethylamine of the 2C family. It was first synthesized in the laboratory of David E. Nichols. It has also been called 2C-CF3, a name derived from the Para-trifluoromethyl group it contains.

2,5-Dimethoxy-4-ethylamphetamine is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book PiHKAL.
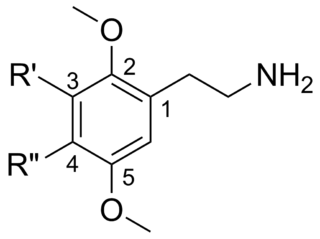
2C (2C-x) is a general name for the family of psychedelic phenethylamines containing methoxy groups on the 2 and 5 positions of a benzene ring. Most of these compounds also carry lipophilic substituents at the 4 position, usually resulting in more potent and more metabolically stable and longer acting compounds. Most of the currently known 2C compounds were first synthesized by Alexander Shulgin in the 1970s and 1980s and published in his book PiHKAL. Shulgin also coined the term 2C, being an acronym for the 2 carbon atoms between the benzene ring and the amino group.

Aleph is a psychedelic hallucinogenic drug and a substituted amphetamine of the phenethylamine class of compounds, which can be used as an entheogen. It was first synthesized by Alexander Shulgin, who named it after the first letter of the Hebrew alphabet. In his book PiHKAL, Shulgin lists the dosage range as 5–10 mg, with effects typically lasting for 6 to 8 hours.

2C-T-13 is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL.

2C-T-15 or 2,5-dimethoxy-4-(β-cyclopropylthio)phenethylamine is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL .
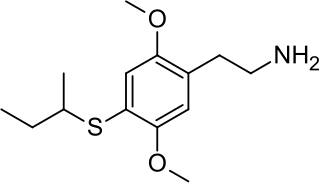
2C-T-17 or 2,5-dimethoxy-4-(β-secbutylthio)phenethylamine is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL .

2,5-Dimethoxy-4-butylamphetamine (DOBU) is a lesser-known psychedelic drug and a substituted amphetamine. DOBU was first synthesized by Alexander Shulgin. In his book PiHKAL (Phenethylamines i Have Known And Loved), only low dosages of 2–3 mg were tested, with the duration simply listed as "very long". DOBU produces paresthesia and difficulty sleeping, but with few other effects. Compared to shorter chain homologues such as DOM, DOET and DOPR which are all potent hallucinogens, DOBU has an even stronger 5-HT2 binding affinity but fails to substitute for hallucinogens in animals or produce hallucinogenic effects in humans, suggesting it has low efficacy and is thus an antagonist or weak partial agonist at the 5-HT2A receptor.

Dimethoxy-4-amylamphetamine (DOAM) is a lesser-known psychedelic drug and a substituted amphetamine. DOAM was first synthesized by Alexander Shulgin. In his book PiHKAL (Phenethylamines i Have Known And Loved), the minimum dosage is listed as 10 mg, and the duration is unknown. DOAM produces a bare threshold and tenseness. As the 4-alkyl chain length is increased from shorter homologues such as DOM, DOET and DOPR which are all potent hallucinogens, the 5-HT2 binding affinity increases, rising to a maximum with the 4-(n-hexyl) derivative before falling again with even longer chains, but compounds with chain length longer than n-propyl, or with other bulky groups such as isopropyl, t-butyl or γ-phenylpropyl at the 4- position, fail to substitute for hallucinogens in animals or produce hallucinogenic effects in humans, suggesting these have low efficacy and are thus antagonists or partial agonists at the 5-HT2A receptor.

2,5-Dimethoxy-4-ethoxyamphetamine (MEM) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin. In his book PiHKAL, he lists the active dose range as 20–50 mg, and the duration as 10–14 hours. According to Shulgin, MEM produces color enhancement, visual phenomena, and pattern movement, among other effects.
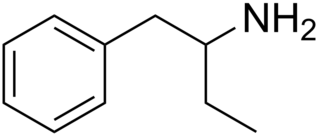
Phenylisobutylamine, also known as α-ethylphenethylamine, Butanphenamine, B or AEPEA, is a stimulant drug of the phenethylamine class. It is a higher homologue of amphetamine, differing from amphetamine's molecular structure only by the substitution of the methyl group at the alpha position of the side chain with an ethyl group.

2,5-Dimethoxy-4-fluoroamphetamine (DOF) is a psychedelic drug of the phenethylamine and amphetamine classes. Alexander Shulgin briefly describes DOF in his book PiHKAL:
Animal studies that have compared DOF to the highly potent DOI and DOB imply that the human activity will be some four to six times less than these two heavier halide analogues.

4C-B is a lesser-known psychedelic drug which is related to 2C-B and DOB. It is a reasonably potent 5-HT2A receptor partial agonist with a Ki of 7.6nM, but has relatively low efficacy. It is briefly mentioned in Alexander Shulgin's book PiHKAL but was never tested by him, however it has subsequently been tested by other researchers and was found to be active in a dose range of 50-80mg with a duration of around 8 hours, though with generally milder effects than 2C-B or DOB.




















