
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom (=N−). It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
Extractive metallurgy is a branch of metallurgical engineering wherein process and methods of extraction of metals from their natural mineral deposits are studied. The field is a materials science, covering all aspects of the types of ore, washing, concentration, separation, chemical processes and extraction of pure metal and their alloying to suit various applications, sometimes for direct use as a finished product, but more often in a form that requires further working to achieve the given properties to suit the applications.

Pyridinium refers to the cation [C5H5NH]+. It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.

Bromoform is an organic compound with the chemical formula CHBr3. It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is very slightly soluble in water and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils.

Copper(II) chloride, also known as cupric chloride, is an inorganic compound with the chemical formula CuCl2. The monoclinic yellowish-brown anhydrous form slowly absorbs moisture to form the orthorhombic blue-green dihydrate CuCl2·2H2O, with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.
Ruthenium tetroxide is the inorganic compound with the formula RuO4. It is a yellow volatile solid that melts near room temperature. It has the odor of ozone. Samples are typically black due to impurities. The analogous OsO4 is more widely used and better known. It is also the anhydride of hyperruthenic acid (H2RuO5). One of the few solvents in which RuO4 forms stable solutions is CCl4.

Rosocyanine and rubrocurcumin are two red colored materials, which are formed by the reaction between curcumin and borates.

The Zincke–Suhl reaction is a special case of a Friedel-Crafts alkylation and was first described by Theodor Zincke and Suhl in 1906. Unlike the traditional Friedel-Crafts reaction, the reduction of the phenyl ring leads to a higher energy final product that can be used as starting material in the dienol–benzene rearrangement, among other reactions.

Desoxypipradrol, also known as 2-diphenylmethylpiperidine (2-DPMP), is a drug developed by Ciba in the 1950s which acts as a norepinephrine-dopamine reuptake inhibitor (NDRI).
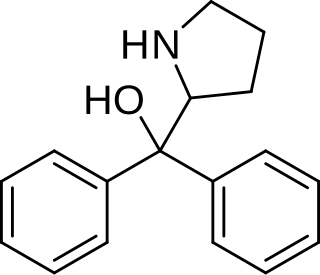
Diphenylprolinol (D2PM), or (R/S)-(±)-diphenyl-2-pyrrolidinyl-methanol, is a norepinephrine-dopamine reuptake inhibitor which is used as a designer drug.

Chloroauric acid is an inorganic compound with the chemical formula H[AuCl4]. It forms hydrates H[AuCl4]·nH2O. Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar [AuCl4]− anion. Often chloroauric acid is handled as a solution, such as those obtained by dissolution of gold in aqua regia. These solutions can be converted to other gold complexes or reduced to metallic gold or gold nanoparticles.
In chemistry, ion association is a chemical reaction whereby ions of opposite electric charge come together in solution to form a distinct chemical entity. Ion associates are classified, according to the number of ions that associate with each other, as ion pairs, ion triplets, etc. Ion pairs are also classified according to the nature of the interaction as contact, solvent-shared or solvent-separated. The most important factor to determine the extent of ion association is the dielectric constant of the solvent. Ion associates have been characterized by means of vibrational spectroscopy, as introduced by Niels Bjerrum, and dielectric-loss spectroscopy.
(Benzene)chromium tricarbonyl is an organometallic compound with the formula Cr(C6H6)(CO)3. This yellow crystalline solid compound is soluble in common nonpolar organic solvents. The molecule adopts a geometry known as “piano stool” because of the planar arrangement of the aryl group and the presence of three CO ligands as "legs" on the chromium-bond axis.
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.

2-Mercaptopyridine is an organosulfur compound with the formula HSC5H4N. This yellow crystalline solid is a derivative of pyridine. The compound and its derivatives serve primarily as acylating agents. A few of 2-mercaptopyridine's other uses include serving as a protecting group for amines and imides as well as forming a selective reducing agent. 2-Mercaptopyridine oxidizes to [[2,2′-dipyridyl disulfide]].
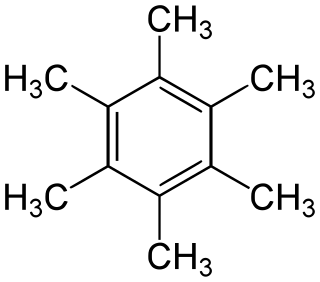
Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.
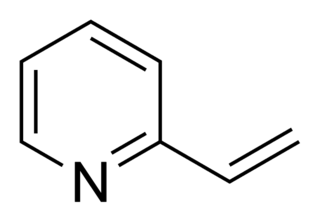
2-Vinylpyridine is an organic compound with the formula CH2CHC5H4N. It is a derivative of pyridine with a vinyl group in the 2-position, next to the nitrogen. It is a colorless liquid, although samples are often brown. It is used industrially as a precursor to specialty polymers and as an intermediate in the chemical, pharmaceutical, dye, and photo industries. Vinylpyridine is sensitive to polymerization. It may be stabilized with a polymerisation inhibitor such as tert-butylcatechol. Owing to its tendency to polymerize, samples are typically refrigerated.
Astatine compounds are compounds that contain the element astatine (At). As this element is very radioactive, few compounds have been studied. Less reactive than iodine, astatine is the least reactive of the halogens. Its compounds have been synthesized in nano-scale amounts and studied as intensively as possible before their radioactive disintegration. The reactions involved have been typically tested with dilute solutions of astatine mixed with larger amounts of iodine. Acting as a carrier, the iodine ensures there is sufficient material for laboratory techniques to work. Like iodine, astatine has been shown to adopt odd-numbered oxidation states ranging from −1 to +7.

Terephthalaldehyde (TA) is an organic compound with the formula C6H4(CHO)2. It is one of three isomers of benzene dicarboxaldehyde, in which the aldehyde moieties are positioned in the para conformation on the benzene ring. Terephthalaldehyde appears as a white to beige solid, typically in the form of a powder. It is soluble in many organic solvents, such as alcohols (e.g., methanol or ethanol) and ethers (e.g., tetrahydrofuran or diethylether).














