Contents
- Synthesis
- History
- Regulation
- Toxicology
- Incidents
- James River estuary
- French Antilles
- References
- External links
 | |
 | |
| Names | |
|---|---|
| IUPAC name decachloropentacyclo[5.3.0.02.6.03.9.04.8]decan-5-one [1] | |
| Other names Chlordecone Clordecone Merex CAS name: 1,1a,3,3a,4,5,5,5a,5b,6-decachlorooctahydro-1,3,4-metheno-2H-cyclobuta[cd]pentalen-2-one | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.093 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
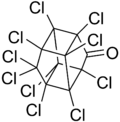
| C10Cl10O | |
| Molar mass | 490.633 g/mol |
| Appearance | tan to white crystalline solid |
| Odor | odorless |
| Density | 1.6 g/cm3 |
| Melting point | 349 °C (660 °F; 622 K) (decomposes) |
| 0.27 g/100 mL | |
| Solubility | soluble in acetone, ketone, acetic acid slightly soluble in benzene, hexane |
| log P | 5.41 |
| Vapor pressure | 3.10−7 kPa |
| Thermochemistry | |
Std molar entropy (S⦵298) | 764 J/K mol |
Std enthalpy of formation (ΔfH⦵298) | −225.9 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | carcinogen [2] |
| Flash point | Non-flammable [2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 95 mg/kg (rat, oral) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | none [2] |
REL (Recommended) | Ca TWA 0.001 mg/m3 [2] |
IDLH (Immediate danger) | N.D. [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Chlordecone, better known in the United States under the brand name Kepone, is an organochlorine compound and a colourless solid. It is an obsolete insecticide, now prohibited in the Western world, but only after many thousands of tonnes had been produced and used. [3] Chlordecone is a known persistent organic pollutant that was banned globally by the Stockholm Convention on Persistent Organic Pollutants in 2009. [4]