
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN, and TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of taste, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.
Mucolipidosis type IV is an autosomal recessive lysosomal storage disorder. Individuals with the disorder have many symptoms including delayed psychomotor development and various ocular aberrations. The disorder is caused by mutations in the MCOLN1 gene, which encodes a non-selective cation channel, mucolipin1. These mutations disrupt cellular functions and lead to a neurodevelopmental disorder through an unknown mechanism. Researchers dispute the physiological role of the protein product and which ion it transports.
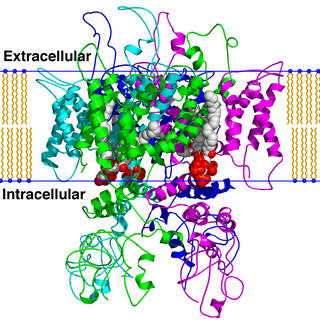
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.

TRPA is a family of transient receptor potential ion channels. The TRPA family is made up of 7 subfamilies: TRPA1, TRPA- or TRPA1-like, TRPA5, painless, pyrexia, waterwitch, and HsTRPA. TRPA1 is the only subfamily widely expressed across animals, while the other subfamilies are largely absent in deuterostomes.

Transient receptor potential cation channel, subfamily C, member 6 or Transient receptor potential canonical 6, also known as TRPC6, is a protein encoded in the human by the TRPC6 gene. TRPC6 is a transient receptor potential channel of the classical TRPC subfamily.

Transient receptor potential canonical 1 (TRPC1) is a protein that in humans is encoded by the TRPC1 gene.

The short transient receptor potential channel 4 (TrpC4), also known as Trp-related protein 4, is a protein that in humans is encoded by the TRPC4 gene.

Short transient receptor potential channel 5 (TrpC5) also known as transient receptor protein 5 (TRP-5) is a protein that in humans is encoded by the TRPC5 gene. TrpC5 is subtype of the TRPC family of mammalian transient receptor potential ion channels.

Transient receptor potential cation channel, subfamily M, member 2, also known as TRPM2, is a protein that in humans is encoded by the TRPM2 gene.

Transient receptor potential cation channel subfamily M member 5 (TRPM5), also known as long transient receptor potential channel 5 is a protein that in humans is encoded by the TRPM5 gene.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

Transient receptor potential cation channel subfamily M member 4 (hTRPM4), also known as melastatin-4, is a protein that in humans is encoded by the TRPM4 gene.

Transient receptor potential cation channel subfamily M member 3 is a protein that in humans is encoded by the TRPM3 gene.

Transient receptor potential cation channel, subfamily V, member 3, also known as TRPV3, is a human gene encoding the protein of the same name.

Transient receptor potential cation channel, subfamily M, member 7, also known as TRPM7, is a human gene encoding a protein of the same name.

Mucolipin-2 also known as TRPML2 is a protein that in humans is encoded by the MCOLN2 gene. It is a member of the small family of the TRPML channels, a subgroup of the large protein family of TRP ion channels.

Mucolipin-3 also known as TRPML3 is a protein that in humans is encoded by the MCOLN3 gene. It is a member of the small family of the TRPML channels, a subgroup of the large protein family of TRP ion channels.
The transient receptor potential Ca2+ channel (TRP-CC) family (TC# 1.A.4) is a member of the voltage-gated ion channel (VIC) superfamily and consists of cation channels conserved from worms to humans. The TRP-CC family also consists of seven subfamilies (TRPC, TRPV, TRPM, TRPN, TRPA, TRPP, and TRPML) based on their amino acid sequence homology:
- the canonical or classic TRPs,
- the vanilloid receptor TRPs,
- the melastatin or long TRPs,
- ankyrin (whose only member is the transmembrane protein 1 [TRPA1])
- TRPN after the nonmechanoreceptor potential C (nonpC), and the more distant cousins,
- the polycystins
- and mucolipins.

MK6-83 is a chemical compound which acts as a channel opener for the TRPML family of calcium channels, with moderate selectivity for TRPML1 over the related TRPML2 and TRPML3 subtypes.



















