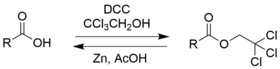Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation, and charge of the molecule. Polyacrylamide gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.

The western blot, or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination.

Chloral hydrate is a geminal diol with the formula Cl3C−CH(OH)2. It was first used as a sedative and hypnotic in Germany in the 1870s. Over time it was replaced by safer and more effective alternatives but it remained in usage in the United States until at least the 1970s. It sometimes finds usage as a laboratory chemical reagent and precursor. It is derived from chloral (trichloroacetaldehyde) by the addition of one equivalent of water.

In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by Albert Coons in 1941. However, immunostaining now encompasses a broad range of techniques used in histology, cell biology, and molecular biology that use antibody-based staining methods.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.

Protein electrophoresis is a method for analysing the proteins in a fluid or an extract. The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium, namely agarose or polyacrylamide. Variants of gel electrophoresis include SDS-PAGE, free-flow electrophoresis, electrofocusing, isotachophoresis, affinity electrophoresis, immunoelectrophoresis, counterelectrophoresis, and capillary electrophoresis. Each variant has many subtypes with individual advantages and limitations. Gel electrophoresis is often performed in combination with electroblotting or immunoblotting to give additional information about a specific protein.
Coomassie brilliant blue is the name of two similar triphenylmethane dyes that were developed for use in the textile industry but are now commonly used for staining proteins in analytical biochemistry. Coomassie brilliant blue G-250 differs from Coomassie brilliant blue R-250 by the addition of two methyl groups. The name "Coomassie" is a registered trademark of Imperial Chemical Industries.

Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.

Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33,342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similar excitation–emission spectra.
Nile red is a lipophilic stain. Nile red stains intracellular lipid droplets yellow. In most polar solvents, Nile red will not fluoresce; however, when in a lipid-rich environment, it can be intensely fluorescent, with varying colors from deep red to strong yellow-gold emission. The dye is highly solvatochromic and its emission and excitation wavelength both shift depending on solvent polarity and in polar media will hardly fluoresce at all.
In pathology, silver staining is the use of silver to selectively alter the appearance of a target in microscopy of histological sections; in temperature gradient gel electrophoresis; and in polyacrylamide gels.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.
Bromocresol green (BCG) is a dye of the triphenylmethane family. It belongs to a class of dyes called sulfonephthaleins. It is used as a pH indicator in applications such as growth mediums for microorganisms and titrations. In clinical practise, it is commonly used as a diagnostic technique. The most common use of bromocresol green is to measure serum albumin concentration within mammalian blood samples in possible cases of kidney failure and liver disease. In chemistry, bromocresol green is used in Thin-layer chromatography staining solutions to visualize acidic compounds.

Tetrasodium tris(bathophenanthroline disulfonate)ruthenium(II) (Na4Ru(bps)3) is a sodium salt of coordination compound. In this form, it is the salt of a sulfonic acid. This compound is an extension of the phenanthroline series of coordination compounds. Ruthenium(II) tris(bathophenanthroline disulfonate), referring to the anionic fragment, is used as a protein dye in biochemistry for differentiating and detecting different proteins in laboratory settings.

An electrophoretic color marker is a chemical used to monitor the progress of agarose gel electrophoresis and polyacrylamide gel electrophoresis (PAGE) since DNA, RNA, and most proteins are colourless. The color markers are made up of a mixture of dyes that migrate through the gel matrix alongside the sample of interest. They are typically designed to have different mobilities from the sample components and to generate colored bands that can be used to assess the migration and separation of sample components.

Fluorescence is used in the life sciences generally as a non-destructive way of tracking or analysing biological molecules. Some proteins or small molecules in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence. Alternatively, specific or general proteins, nucleic acids, lipids or small molecules can be "labelled" with an extrinsic fluorophore, a fluorescent dye which can be a small molecule, protein or quantum dot. Several techniques exist to exploit additional properties of fluorophores, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in properties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.

Epicocconone is a long Stokes' shift fluorogenic natural product found in the fungus Epicoccum nigrum. Though weakly fluorescent in water it reacts covalently yet reversibly with primary amines such as those in proteins to yield a product with a strong orange-red emission (610 nm). Epicoconone is notable because it the first covalent/reversible/turn-on fluorophore to be discovered and is a natural product with a new fluorescent scaffold. It is also cell membrane permeable, unlike many other fluorophores, and subsequently can be used in in vivo applications. Additionally, this dye can be used as a sensitive total protein stain for 1D and 2D electrophoresis, quantitative determination of protein concentration, making it a powerful loading control for Western blots.

2,2,2-Tribromoethanol, often called just tribromoethanol, is a chemical compound with formula Br3C−CH2OH. Its molecule can be described as that of ethanol, with the three hydrogen atoms in position 2 replaced by bromine. It is a white crystalline solid, soluble in water and other solvents, that absorbs strongly in the UV below 290 nm.
Normalization of Western blot data is an analytical step that is performed to compare the relative abundance of a specific protein across the lanes of a blot or gel under diverse experimental treatments, or across tissues or developmental stages. The overall goal of normalization is to minimize effects arising from variations in experimental errors, such as inconsistent sample preparation, unequal sample loading across gel lanes, or uneven protein transfer, which can compromise the conclusions that can be obtained from Western blot data. Currently, there are two methods for normalizing Western blot data: (i) housekeeping protein normalization and (ii) total protein normalization.
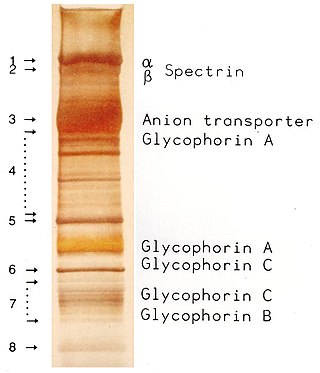
SDS-PAGE is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. The combined use of sodium dodecyl sulfate and polyacrylamide gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall.