Related Research Articles

The insulin-like growth factors (IGFs) are proteins with high sequence similarity to insulin. IGFs are part of a complex system that cells use to communicate with their physiologic environment. This complex system consists of two cell-surface receptors, two ligands, a family of seven high-affinity IGF-binding proteins, as well as associated IGFBP degrading enzymes, referred to collectively as proteases.

The insulin receptor (IR) is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of receptor tyrosine kinase. Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis, a functional process that under degenerate conditions may result in a range of clinical manifestations including diabetes and cancer. Insulin signalling controls access to blood glucose in body cells. When insulin falls, especially in those with high insulin sensitivity, body cells begin only to have access to lipids that do not require transport across the membrane. So, in this way, insulin is the key regulator of fat metabolism as well. Biochemically, the insulin receptor is encoded by a single gene INSR, from which alternate splicing during transcription results in either IR-A or IR-B isoforms. Downstream post-translational events of either isoform result in the formation of a proteolytically cleaved α and β subunit, which upon combination are ultimately capable of homo or hetero-dimerisation to produce the ≈320 kDa disulfide-linked transmembrane insulin receptor.

Insulin-like growth factor 1 (IGF-1), also called somatomedin C, is a hormone similar in molecular structure to insulin which plays an important role in childhood growth, and has anabolic effects in adults.

Acanthosis nigricans is a medical sign characterised by brown-to-black, poorly defined, velvety hyperpigmentation of the skin. It is usually found in body folds, such as the posterior and lateral folds of the neck, the armpits, groin, navel, forehead and other areas.
The DAF-2 gene encodes for the insulin-like growth factor 1 (IGF-1) receptor in the worm Caenorhabditis elegans. DAF-2 is part of the first metabolic pathway discovered to regulate the rate of aging. DAF-2 is also known to regulate reproductive development, resistance to oxidative stress, thermotolerance, resistance to hypoxia, and resistance to bacterial pathogens. Mutations in DAF-2 have been shown by Cynthia Kenyon to double the lifespan of the worms. In a 2007 episode of WNYC’s Radiolab, Kenyon called DAF-2 "the grim reaper gene.”
![Insulin-like growth factor 2 Major fetal growth factor in contrast to Insulin-like growth factor 1, which is a major growth factor in adults."[5]](https://upload.wikimedia.org/wikipedia/commons/thumb/9/92/Protein_IGF2_PDB_1igl.png/320px-Protein_IGF2_PDB_1igl.png)
Insulin-like growth factor 2 (IGF-2) is one of three protein hormones that share structural similarity to insulin. The MeSH definition reads: "A well-characterized neutral peptide believed to be secreted by the liver and to circulate in the blood. It has growth-regulating, insulin-like and mitogenic activities. The growth factor has a major, but not absolute, dependence on somatotropin. It is believed to be a major fetal growth factor in contrast to Insulin-like growth factor 1, which is a major growth factor in adults."

The insulin-like growth factor 1 (IGF-1) receptor is a protein found on the surface of human cells. It is a transmembrane receptor that is activated by a hormone called insulin-like growth factor 1 (IGF-1) and by a related hormone called IGF-2. It belongs to the large class of tyrosine kinase receptors. This receptor mediates the effects of IGF-1, which is a polypeptide protein hormone similar in molecular structure to insulin. IGF-1 plays an important role in growth and continues to have anabolic effects in adults – meaning that it can induce hypertrophy of skeletal muscle and other target tissues. Mice lacking the IGF-1 receptor die late in development, and show a dramatic reduction in body mass. This testifies to the strong growth-promoting effect of this receptor.
A somatomedinreceptor is a receptor which binds the somatomedins (IGFs). Somatomedin is abbreviated to IGF, in reference to insulin-like growth factor.

The insulin-like growth factor-binding protein (IGFBP) serves as a transport protein for insulin-like growth factor 1 (IGF-1).

Insulin-like growth factor 2 receptor (IGF2R), also called the cation-independent mannose-6-phosphate receptor (CI-MPR) is a protein that in humans is encoded by the IGF2R gene. IGF2R is a multifunctional protein receptor that binds insulin-like growth factor 2 (IGF2) at the cell surface and mannose-6-phosphate (M6P)-tagged proteins in the trans-Golgi network.
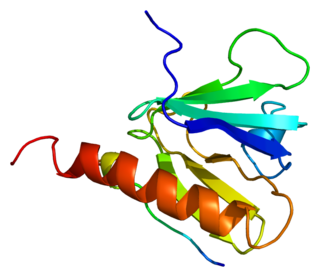
Insulin receptor substrate 1 (IRS-1) is a signaling adapter protein that in humans is encoded by the IRS-1 gene. It is a 131 kDa protein with amino acid sequence of 1242 residues. It contains a single pleckstrin homology (PH) domain at the N-terminus and a PTB domain ca. 40 residues downstream of this, followed by a poorly conserved C-terminus tail. Together with IRS2, IRS3 (pseudogene) and IRS4, it is homologous to the Drosophila protein chico, whose disruption extends the median lifespan of flies up to 48%. Similarly, Irs1 mutant mice experience moderate life extension and delayed age-related pathologies.

Insulin-like growth factor-binding protein 3, also known as IGFBP-3, is a protein that in humans is encoded by the IGFBP3 gene. IGFBP-3 is one of six IGF binding proteins that have highly conserved structures and bind the insulin-like growth factors IGF-1 and IGF-2 with high affinity. IGFBP-7, sometimes included in this family, shares neither the conserved structural features nor the high IGF affinity. Instead, IGFBP-7 binds IGF1R, which blocks IGF-1 and IGF-2 binding, resulting in apoptosis.

Insulin-like growth factor-binding protein 5 is a protein that in humans is encoded by the IGFBP5 gene. An IGFBP5 gene was recently identified as being important for adaptation to varying water salinity in fish.

Insulin-like growth factor-binding protein 4 is a protein that in humans is encoded by the IGFBP4 gene.

Insulin-like growth factor-binding protein 1 (IBP-1) also known as placental protein 12 (PP12) is a protein that in humans is encoded by the IGFBP1 gene.

Insulin-like growth factor-binding protein 7 is a protein that in humans is encoded by the IGFBP7 gene. The major function of the protein is the regulation of availability of insulin-like growth factors (IGFs) in tissue as well as in modulating IGF binding to its receptors. IGFBP7 binds to IGF with high affinity. It also stimulates cell adhesion. The protein is implicated in some cancers.
Mecasermin (INN), also known as recombinant human insulin-like growth factor-1 (rhIGF-1), is a recombinant form of human insulin-like growth factor 1 (IGF-I) which is used in the long-term treatment of growth failure and short stature in children with severe primary IGF-I deficiency, for instance due to growth hormone deficiency or Laron syndrome.
Figitumumab is a monoclonal antibody targeting the insulin-like growth factor-1 receptor that was investigated for the treatment of various types of cancer, for example adrenocortical carcinoma and non-small cell lung cancer (NSCLC).
Breast development, also known as mammogenesis, is a complex biological process in primates that takes place throughout a female's life.
Long arginine 3-IGF-1, abbreviated as IGF-1 LR3 or LR3-IGF-1, is a synthetic protein and lengthened analogue of human insulin-like growth factor 1 (IGF-1). It differs from native IGF-1 in that it possesses an arginine instead of a glutamic acid at the third position in its amino acid sequence, and also has an additional 13 amino acids at its N-terminus (MFPAMPLLSLFVN) ("long"), for a total of 83 amino acids. The consequences of these modifications are that IGF-1 LR3 retains the pharmacological activity of IGF-1 as an agonist of the IGF-1 receptor, has very low affinity for the insulin-like growth factor-binding proteins (IGFBPs), and has improved metabolic stability. As a result, it is approximately three times more potent than IGF-1, and possesses a significantly longer half-life of about 20–30 hours.