
Platelets or thrombocytes are a blood component whose function is to react to bleeding from blood vessel injury by clumping, thereby initiating a blood clot. Platelets have no cell nucleus; they are fragments of cytoplasm derived from the megakaryocytes of the bone marrow or lung, which then enter the circulation. Platelets are found only in mammals, whereas in other vertebrates, thrombocytes circulate as intact mononuclear cells.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The process of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Prothrombin is encoded in the human by the F2-gene. It is proteolytically cleaved during the clotting process by the prothrombinase enzyme complex to form thrombin.
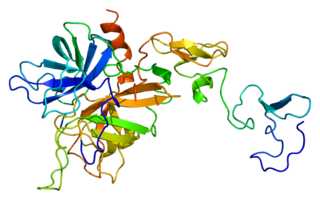
Protein C, also known as autoprothrombin IIA and blood coagulation factor XIV, is a zymogen, that is, an inactive enzyme. The activated form plays an important role in regulating anticoagulation, inflammation, and cell death and maintaining the permeability of blood vessel walls in humans and other animals. Activated protein C (APC) performs these operations primarily by proteolytically inactivating proteins Factor Va and Factor VIIIa. APC is classified as a serine protease since it contains a residue of serine in its active site. In humans, protein C is encoded by the PROC gene, which is found on chromosome 2.

Coagulation factor X, or Stuart factor, is an enzyme of the coagulation cascade, encoded in humans by F10 gene. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.
Protease-activated receptors (PAR) are a subfamily of related G protein-coupled receptors that are activated by cleavage of part of their extracellular domain. They are highly expressed in platelets, and also on endothelial cells, fibroblasts, immune cells, myocytes, neurons, and tissues that line the gastrointestinal tract.
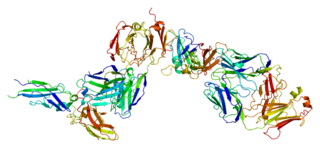
Tissue factor, also called platelet tissue factor or Coagulation factor III, is a protein present in subendothelial tissue and leukocytes which plays a major role in coagulation and, in humans, is encoded by F3 gene. Its role in the blood clotting is the initiation of thrombin formation from the zymogen prothrombin. Thromboplastin defines the cascade that leads to the activation of factor X—the tissue factor pathway. In doing so, it has replaced the previously named extrinsic pathway in order to eliminate ambiguity.

P-selectin is a type-1 transmembrane protein that in humans is encoded by the SELP gene.
The actions of vasopressin are mediated by stimulation of tissue-specific G protein-coupled receptors (GPCRs) called vasopressin receptors that are classified into the V1 (V1A), V2, and V3 (V1B) receptor subtypes. These three subtypes differ in localization, function and signal transduction mechanisms.
Kininogens are precursor proteins for kinins, biologically active polypeptides involved in blood coagulation, vasodilation, smooth muscle contraction, inflammatory regulation, and the regulation of the cardiovascular and renal systems.

The thromboxane receptor (TP) also known as the prostanoid TP receptor is a protein that in humans is encoded by the TBXA2R gene, The thromboxane receptor is one among the five classes of prostanoid receptors and was the first eicosanoid receptor cloned. The TP receptor derives its name from its preferred endogenous ligand thromboxane A2.

G-protein-coupled receptor kinase 2 (GRK2) is an enzyme that in humans is encoded by the ADRBK1 gene. GRK2 was initially called Beta-adrenergic receptor kinase, and is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases that is most highly similar to GRK3(βARK2).

Protease activated receptor 3 (PAR-3) also known as coagulation factor II receptor-like 2 (F2RL2) and thrombin receptor-like 2, is a protein that in humans is encoded by the F2RL2 gene.

Protease activated receptor 2 (PAR2) also known as coagulation factor II (thrombin) receptor-like 1 (F2RL1) or G-protein coupled receptor 11 (GPR11) is a protein that in humans is encoded by the F2RL1 gene. PAR2 modulates inflammatory responses, obesity, metabolism, cancers and acts as a sensor for proteolytic enzymes generated during infection. In humans, we can find PAR2 in the stratum granulosum layer of epidermal keratinocytes. Functional PAR2 is also expressed by several immune cells such as eosinophils, neutrophils, monocytes, macrophages, dendritic cells, mast cells and T cells.

Proteinase-activated receptor 1 (PAR1) also known as protease-activated receptor 1, coagulation factor II receptor and thrombin receptor is a protein that in humans is encoded by the F2R gene. PAR1 is a G protein-coupled receptor and one of four protease-activated receptors involved in the regulation of thrombotic response. Highly expressed in platelets and endothelial cells, PAR1 plays a key role in mediating the interplay between coagulation and inflammation, which is important in the pathogenesis of inflammatory and fibrotic lung diseases. It is also involved both in disruption and maintenance of endothelial barrier integrity, through interaction with either thrombin or activated protein C, respectively.

Protease-activated receptor 4 (PAR-4), also known as coagulation factor II (thrombin) receptor-like 3, is a protein that in humans is encoded by the F2RL3 gene.

Endothelial protein C receptor (EPCR) also known as activated protein C receptor is a protein that in humans is encoded by the PROCR gene. PROCR has also recently been designated CD201.
Pepducins are cell-penetrating peptides that act as intracellular modulators of signal transference from receptors to G proteins. Pepducins were first developed at the Tufts Medical Center laboratories of Dr. Athan Kuliopulos and Dr. Lidija Covic.

SCH-79797 is a drug which acts as a potent and selective antagonist of the thrombin receptor proteinase activated receptor 1 (PAR1). It has anticoagulant, anticonvulsant and antiinflammatory effects and has been researched as a treatment for heart attack and stroke, though never developed for medical use. It also shows antibiotic actions which are not shared with other PAR1 antagonists such as vorapaxar, so may be mediated through a different target than PAR1.













