
Mescaline, also known as mescalin or mezcalin, and in chemical terms 3,4,5-trimethoxyphenethylamine, is a naturally occurring psychedelic protoalkaloid of the substituted phenethylamine class, known for its hallucinogenic effects comparable to those of LSD and psilocybin. It binds to and activates certain serotonin receptors in the brain, producing hallucinogenic effects.

PiHKAL: A Chemical Love Story is a book by Alexander Shulgin and Ann Shulgin, published in 1991. The subject of the work is psychoactive phenethylamine chemical derivatives, notably those that act as psychedelics and/or empathogen-entactogens. The main title, PiHKAL, is an acronym that stands for "Phenethylamines I Have Known and Loved".

3,4-Methylenedioxyamphetamine (MDA), sometimes referred to as sass, is an empathogen-entactogen, stimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
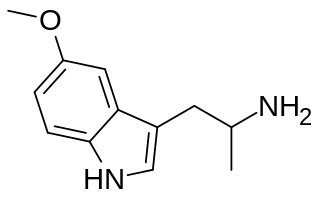
5-MeO-αMT, or 5-methoxy-α-methyltryptamine, also known as α,O-dimethylserotonin (Alpha-O), is a serotonergic psychedelic of the tryptamine family. It is a derivative of α-methyltryptamine (αMT) and an analogue of 5-MeO-DMT.

2,4,5-Trimethoxyamphetamine (2,4,5-TMA), also known as TMA-2 or as 2,5-dimethoxy-4-methoxyamphetamine (DOMeO), is a psychedelic drug of the phenethylamine and amphetamine families. It is one of the trimethoxyamphetamine (TMA) series of positional isomers. The drug is also notable in being the 4-methoxylated member of the DOx series of drugs.
Trimethoxyamphetamines (TMAs) are a family of positionally isomeric psychedelic hallucinogenic drugs. There exist six different TMAs that differ only in the positions of the three methoxy groups: TMA (TMA-1), TMA-2, TMA-3, TMA-4, TMA-5, and TMA-6. The TMAs are substituted amphetamines and are analogues of the phenethylamine cactus alkaloid mescaline and the DOx drugs.

Aminorex, sold under the brand names Menocil and Apiquel among others, is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension (PPH). In the United States, aminorex is a Schedule I controlled substance.
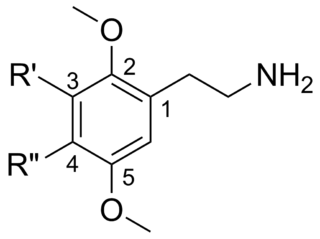
2C (2C-x) is a general name for the family of psychedelic phenethylamines containing methoxy groups on the 2 and 5 positions of a benzene ring. Most of these compounds also carry lipophilic substituents at the 4 position, usually resulting in more potent and more metabolically stable and longer acting compounds.

Aleph is a psychedelic hallucinogenic drug and a substituted amphetamine of the phenethylamine class of compounds, which can be used as an entheogen. It was first synthesized by Alexander Shulgin, who named it after the first letter of the Hebrew alphabet. In his book PiHKAL, Shulgin lists the dosage range as 5–10 mg, with effects typically lasting for 6 to 8 hours.

3,4-Dimethoxyphenethylamine (DMPEA) is a chemical compound of the phenethylamine class. It is an analogue of the major human neurotransmitter dopamine where the 3- and 4-position hydroxy groups have been replaced with methoxy groups. It is also closely related to mescaline which is 3,4,5-trimethoxyphenethylamine and to 3,4-dimethoxyamphetamine (3,4-DMA).
Dimethoxyamphetamine (DMA) is a series of six lesser-known psychedelic drugs similar in structure to the three isomers of methoxyamphetamine and six isomers of trimethoxyamphetamine. The isomers are 2,3-DMA, 2,4-DMA, 2,5-DMA, 2,6-DMA, 3,4-DMA, and 3,5-DMA. Three of the isomers were characterized by Alexander Shulgin in his book PiHKAL. Little is known about their dangers or toxicity.

Etilamfetamine, also known as N-ethylamphetamine and formerly sold under the brand names Apetinil and Adiparthrol, is a stimulant drug of the amphetamine family. It was invented in the early 20th century and was subsequently used as an anorectic or appetite suppressant in the 1950s, but was not as commonly used as other amphetamines such as amphetamine, methamphetamine, and benzphetamine, and was largely discontinued once newer drugs such as phenmetrazine were introduced.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) and serotonergic neurotoxin of the amphetamine family. It is used in scientific research in the study of the serotonin system, as a serotonin releasing agent (SRA) at lower doses to produce serotonergic effects, and as a serotonergic neurotoxin at higher doses to produce long-lasting depletions of serotonin.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; MRAs can induce the release of one or more of these neurotransmitters.

3-Methoxyamphetamine (3-MA), also known as meta-methoxyamphetamine (MMA), is a monoamine releasing agent (MRA) of the amphetamine family. It is a positional isomer of para-methoxyamphetamine.

2,5-Dimethoxyamphetamine (2,5-DMA), also known as DMA-4 or as DOH, is a drug of the phenethylamine and amphetamine families. It is one of the dimethoxyamphetamine (DMA) series of positional isomers. The drug is notable in being the parent compound of the DOx (4-substituted-2,5-dimethoxyamphetamine) series of drugs.

4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring. They are 4-substituted derivatives of 2,5-dimethoxyamphetamine.
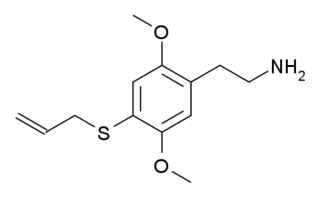
2C-T-16 is a lesser-known psychedelic drug. It was originally named by Alexander Shulgin as described in his book PiHKAL, however while Shulgin began synthesis of this compound he only got as far as the nitrostyrene intermediate, and did not complete the final synthetic step. Synthesis of 2C-T-16 was finally achieved by Daniel Trachsel some years later, and it was subsequently reported as showing similar psychedelic activity to related compounds, with a dose range of 10–25 mg and a duration of 4–6 hours, making it around the same potency as the better-known saturated analogue 2C-T-7, but with a significantly shorter duration of action. Binding studies in vitro showed 2C-T-16 to have a binding affinity of 44 nM at 5-HT2A and 15 nM at 5-HT2C. 2C-T-16 and related derivatives are potent partial agonists of the 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors and induce a head-twitch response in mice.

2C-T-21.5 is a lesser-known psychedelic drug related to compounds such as 2C-T-21 and 2C-T-28. It was originally named by Alexander Shulgin and discussed in his book PiHKAL, but was not synthesised at that time. 2C-T-21.5 was ultimately synthesised and tested by Daniel Trachsel some years later. It has a binding affinity of 146 nM at 5-HT2A and 55 nM at 5-HT2C. It produces typical psychedelic effects, being slightly less potent but somewhat longer acting than 2C-T-2 or 2C-T-21, with an active dose of 12–30 mg, and a duration of action of 8–14 hours. Unlike 2C-T-21 it will not form the highly toxic fluoroacetate as a metabolite, instead producing the less toxic difluoroacetic acid.

3,4-Dimethoxyamphetamine (3,4-DMA), or simply dimethoxyamphetamine (DMA), is a psychedelic drug of the phenethylamine and amphetamine families. It is one of the dimethoxyamphetamine (DMA) series of positional isomers.

















