Sinovac Biotech Ltd. is a Chinese biopharmaceutical company that focuses on the research, development, manufacture and commercialization of vaccines that protect against human infectious diseases. The company is based in Haidian District, Beijing. The company is listed on the NASDAQ but the exchange halted Sinovac's trading in February 2019 due to a proxy fight.

The Execution of Imam Khomeini's Order (EIKO),, also known as the Executive Headquarters of Imam's Directive or simply Setad, is a parastatal in the Islamic Republic of Iran, under direct control of the Supreme Leader of Iran. It was created from thousands of properties confiscated in the aftermath of the 1979 Islamic Revolution. A Reuters investigation found that the organization built "its empire on the systematic seizure of thousands of properties belonging to ordinary Iranians", also seizing property from members of religious minorities, business people and Iranians living abroad; at times falsely claiming that the properties were abandoned.

The Barakat Foundation is connected to Execution of Imam Khomeini's Order for the purpose of helping the underprivileged. According to Reuters, the foundation "is a unit of a massive business empire controlled by Seyyed Ali Khamenei that is known as Setad Ejraiye Farmane Hazrate Emam," which has become one of the most powerful Iranian institutions through the seizure of properties belonging to Iranian citizens.

Barkat Pharmaceutical Group is an Iranian Pharmaceutical public company, which was founded in 2010, named Tadbir innovation pharmaceutical company. The company provides services through cooperation between science-based institutions and scientists based on medicine around the world. It supplies 14 percent of all the country's essential drugs through its 25 subsidiaries. The company produces 700 kinds of products in the pharmaceutical sector and offers it internally. The Barkat Pharmaceutical Group has established as the first specialized drug and pharmaceutical research center in Iran, also it has been developed by constructing advanced pharmaceutical factories, called Barkat Pharmaceutical Town. The Barkat group complies with all common pharmaceutical standards such as the FDA, WHO, EMEA. "Cell therapy", production of "peptide medications", "research, development and processing of medicinal plants," the creation of the "Museum of Iranian-Islamic Medicine" as well as "Solids and Semi-Solids" projects are the main activities of the Group.
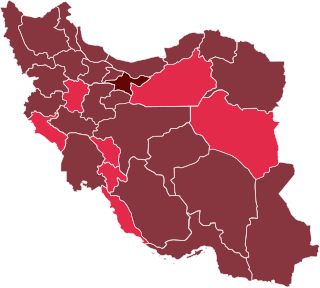
The COVID-19 pandemic in Iran is part of the worldwide pandemic of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2. On 19 February 2020, Iran reported its first confirmed cases of infections in Qom. The virus may have been brought to the country by a merchant from Qom who had travelled to China.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus that causes coronavirus disease 2019 (COVID‑19). Prior to the COVID‑19 pandemic, an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). This knowledge accelerated the development of various vaccine technologies during early 2020. The initial focus of SARS-CoV-2 vaccines was on preventing symptomatic, often severe illness. On 10 January 2020, the SARS-CoV-2 genetic sequence data was shared through GISAID, and by 19 March, the global pharmaceutical industry announced a major commitment to address COVID-19. The COVID‑19 vaccines are widely credited for their role in reducing the spread, severity, and death caused by COVID-19.

The Moderna COVID‑19 vaccine, codenamed mRNA-1273 and sold under the brand name Spikevax, is a COVID-19 vaccine developed by Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA). It is authorized for use in people aged 12 years and older in some jurisdictions and for people 18 years and older in other jurisdictions to provide protection against COVID-19 which is caused by infection by the SARS-CoV-2 virus. It is designed to be administered as two 0.5 mL doses given by intramuscular injection at an interval of 29 days apart.

AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 developed by CanSino Biologics. It conducted its Phase III trials in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.

The Oxford–AstraZeneca COVID-19 vaccine, codenamed AZD1222, and sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for prevention of COVID-19. Developed by Oxford University and AstraZeneca, it is given by intramuscular injection, using as a vector the modified chimpanzee adenovirus ChAdOx1. Studies carried out in 2020 showed that the efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose and 81.3% after the second dose. Another analysis showed that, for symptomatic COVID-19 infection after the second dose, the vaccine is 66% effective against the Alpha variant, and 60% against the Delta variant.

CoronaVac, also known as the Sinovac COVID-19 vaccine, is an inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech. It was Phase III clinical trialled in Brazil, Chile, Indonesia, the Philippines, and Turkey and relies on traditional technology similar to BBIBP-CorV and Covaxin, other inactivated-virus COVID-19 vaccines. CoronaVac does not need to be frozen and both the final product and the raw material for formulating CoronaVac can be transported refrigerated at 2–8 °C (36–46 °F), temperatures at which flu vaccines are kept.
Barkat Ventures is an Iranian knowledge-based institute which has been established as the arm of the Imam (Khomeini)'s Command's Executive in order to develop knowledge-based economy. This institute's mission is to create and expand on the ecosystem/infrastructure for the development of science and knowledge-based activities of the scholars in Iran on the basis of Islamic-Iranian models by utilizing Iranian scientists, experts, global-experience, knowledge and so on.

The Novavax COVID-19 vaccine, codenamed NVX-CoV2373, is a subunit COVID-19 vaccine candidate developed by Novavax and the Coalition for Epidemic Preparedness Innovations (CEPI), that is undergoing trials in India under the brand name Covovax. It requires two doses and is stable at 2 to 8 °C refrigerated temperatures.

India began administration of COVID-19 vaccines on 16 January 2021. As of 25 July 2021, India has administered over 435 million doses overall, including first and second doses of the currently-approved vaccines.

COVID-19's caused virus, SARS-CoV-2, was isolated in late 2019. Its genetic sequence was published on 11 January 2020, triggering an urgent international response to prepare for an outbreak and hasten development of a preventive COVID-19 vaccine. Since 2020, vaccine development has been expedited via unprecedented collaboration in the multinational pharmaceutical industry and between governments. By June 2020, tens of billions of dollars were invested by corporations, governments, international health organizations, and university research groups to develop dozens of vaccine candidates and prepare for global vaccination programs to immunize against COVID‑19 infection. According to the Coalition for Epidemic Preparedness Innovations (CEPI), the geographic distribution of COVID‑19 vaccine development shows North American entities to have about 40% of the activity, compared to 30% in Asia and Australia, 26% in Europe, and a few projects in South America and Africa.

Soberana 02 or Soberana 2, technical name FINLAY-FR-2, is a COVID-19 vaccine produced by the Finlay Institute, a Cuban epidemiological research institute. It is a conjugate vaccine. This candidate followed a previous one called SOBERANA-01 (FINLAY-FR-1). Professor Ihosvany Castellanos Santos said that the antigen is safe because it contains parts instead of the whole live virus, and therefore it does not require extra refrigeration, like other candidates in the world. According to the WHO candidate landscape vaccine document, this vaccine requires two doses, the second one being administered 28 days after the first shot.

EpiVacCorona is a peptide-based vaccine against COVID-19 developed by the VECTOR center of Virology. It consists of three chemically synthesized peptides that are conjugated to a large carrier protein. This protein is a fusion product of a viral nucleocapsid protein and a bacterial MBP protein. The third phase of a clinical trial, which should show whether the vaccine is able to protect people from COVID-19 or not, was launched in November 2020 with more than three thousand participants. It is assumed it will be completed in August 2021. According to the vaccine developers, the peptides and the viral part of the chimeric protein should immunize people who received this vaccine against SARS-CoV-2 and trigger the production of protective antibodies. However, some experts in the field have expressed concerns about the selection of peptides for use as vaccine antigens. In addition, there are also serious concerns about the vaccine immunogenicity data, which have fueled independent civic research efforts and criticism by some experts. Meanwhile, the EpiVacCorona has received vaccine emergency authorization in a form of government registration in Russia and is available for vaccination outside the clinical trials. The vaccine delivered via intramuscular route and aluminum hydroxide serves as an immunological adjuvant.

COVID-19 vaccination in South Africa is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.

ZyCoV-D is a DNA plasmid based COVID-19 vaccine being developed by Cadila Healthcare with support from the Biotechnology Industry Research Assistance Council.

Nanocovax is a Vietnamese COVID-19 vaccine candidate developed by Nanogen Pharmaceutical Biotechnology JSC. It is a subunit vaccine.