
Tricyclic antidepressants (TCAs) are a class of medications that are used primarily as antidepressants, which is important for the management of depression. They are second-line drugs next to SSRIs. TCAs were discovered in the early 1950s and were marketed later in the decade. They are named after their chemical structure, which contains three rings of atoms. Tetracyclic antidepressants (TeCAs), which contain four rings of atoms, are a closely related group of antidepressant compounds.

Tramadol, sold under the brand name Ultram among others, is an opioid pain medication used to treat moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen).

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, obsessive–compulsive disorder (OCD), social phobia, attention-deficit hyperactivity disorder (ADHD), chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.

Amoxapine, sold under the brand name Asendin among others, is a tricyclic antidepressant (TCA). It is the N-demethylated metabolite of loxapine. Amoxapine first received marketing approval in the United States in 1980, approximately 10 to 20 years after most of the other TCAs were introduced in the United States.

Noradrenergic and specific serotonergic antidepressants (NaSSAs) are a class of psychiatric drugs used primarily as antidepressants. They act by antagonizing the α2-adrenergic receptor and certain serotonin receptors such as 5-HT2A and 5-HT2C, but also 5-HT3, 5-HT6, and/or 5-HT7 in some cases. By blocking α2-adrenergic autoreceptors and heteroreceptors, NaSSAs enhance adrenergic and serotonergic neurotransmission in the brain involved in mood regulation, notably 5-HT1A-mediated transmission. In addition, due to their blockade of certain serotonin receptors, serotonergic neurotransmission is not facilitated in unwanted areas, which prevents the incidence of many side effects often associated with selective serotonin reuptake inhibitor (SSRI) antidepressants; hence, in part, the "specific serotonergic" label of NaSSAs.

Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression. It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activities such as weak serotonin reuptake inhibitory, α1-blocking, antihistamine, and anticholinergic effects. The drug is not considered a first-line treatment for depression since the introduction of selective serotonin reuptake inhibitor (SSRI) antidepressants, which have fewer side effects and are safer in overdose.

Clomipramine, sold under the brand name Anafranil among others, is a tricyclic antidepressant (TCA). It is used for the treatment of obsessive–compulsive disorder, panic disorder, major depressive disorder, and chronic pain. It may increase the risk of suicide in those under the age of 25. It is primarily taken by mouth. It has also been used to treat premature ejaculation.

Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression. It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively. The drug is described as an atypical or "second-generation" TCA because, unlike other TCAs, it seems to be a fairly weak monoamine reuptake inhibitor. Similarly to other TCAs however, trimipramine does have antihistamine, antiserotonergic, antiadrenergic, antidopaminergic, and anticholinergic activities.

Nefazodone, sold formerly under the brand names Serzone, Dutonin, and Nefadar among others, is an atypical antidepressant medication which is used in the treatment of depression and for other uses. Nefazodone is still available in the United States, but was withdrawn from other countries due to rare liver toxicity. The medication is taken by mouth.

Dosulepin, also known as dothiepin and sold under the brand name Prothiaden among others, is a tricyclic antidepressant (TCA) which is used in the treatment of depression. Dosulepin was once the most frequently prescribed antidepressant in the United Kingdom, but it is no longer widely used due to its relatively high toxicity in overdose without therapeutic advantages over other TCAs. It acts as a serotonin–norepinephrine reuptake inhibitor (SNRI) and also has other activities including antihistamine, antiadrenergic, antiserotonergic, anticholinergic, and sodium channel-blocking effects.
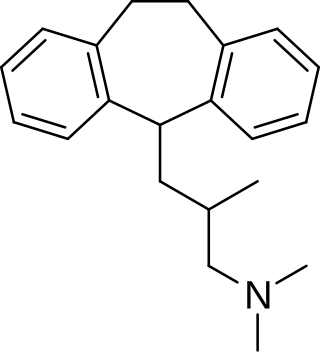
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

Desmetramadol (INN), also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with low CYP2D6 activity unlike tramadol.
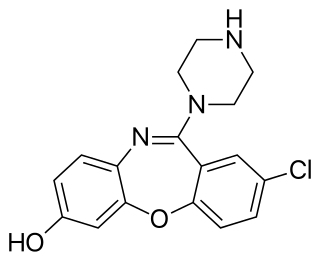
7-Hydroxyamoxapine is an active metabolite of the antidepressant drug amoxapine (Asendin). It contributes to amoxapine's pharmacology. It is a dopamine receptor antagonist and contributes to amoxapine's antipsychotic properties.

Medifoxamine, previously sold under the brand names Clédial and Gerdaxyl, is an atypical antidepressant with additional anxiolytic properties acting via dopaminergic and serotonergic mechanisms which was formerly marketed in France and Spain, as well as Morocco. The drug was first introduced in France sometime around 1990. It was withdrawn from the market in 1999 (Morocco) and 2000 (France) following incidences of hepatotoxicity.
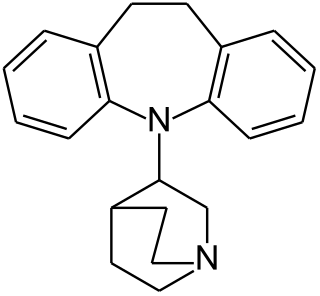
Quinupramine is a tricyclic antidepressant (TCA) used in Europe for the treatment of depression.

Mepiprazole is an anxiolytic drug of the phenylpiperazine group with additional antidepressant properties that is marketed in Spain. It acts as a 5-HT2A and α1-adrenergic receptor antagonist and inhibits the reuptake and induces the release of serotonin, dopamine, and norepinephrine to varying extents, and has been described as a serotonin antagonist and reuptake inhibitor (SARI). Controlled clinical trials of mepiprazole in patients with irritable bowel syndrome (IBS) were also carried out and suggested some benefits of the drug in relieving symptoms of IBS in some patients. Similarly to other phenylpiperazines like trazodone, nefazodone, and etoperidone, mepiprazole produces mCPP as an active metabolite.

A serotonin–dopamine reuptake inhibitor (SDRI) is a type of drug which acts as a reuptake inhibitor of the monoamine neurotransmitters serotonin and dopamine by blocking the actions of the serotonin transporter (SERT) and dopamine transporter (DAT), respectively. This in turn leads to increased extracellular concentrations of serotonin and dopamine, and, therefore, an increase in serotonergic and dopaminergic neurotransmission.

Levofenfluramine (INN), or (−)-3-trifluoromethyl-N-ethylamphetamine, also known as (−)-fenfluramine or (R)-fenfluramine, is a drug of the amphetamine family that, itself (i.e., in enantiopure form), was never marketed. It is the levorotatory enantiomer of fenfluramine, the racemic form of the compound, whereas the dextrorotatory enantiomer is dexfenfluramine. Both fenfluramine and dexfenfluramine are anorectic agents that have been used clinically in the treatment of obesity (and hence, levofenfluramine has been as well since it is a component of fenfluramine). However, they have since been discontinued due to reports of causing cardiovascular conditions such as valvular heart disease and pulmonary hypertension, adverse effects that are likely to be caused by excessive stimulation of 5-HT2B receptors expressed on heart valves.

Triazoledione is a phenylpiperazine compound and a major metabolite of the antidepressant nefazodone. It is active, but with substantially reduced potency compared to nefazodone. As such, it has been suggested that it is unlikely that triazoledione contributes significantly to the pharmacology of nefazodone. However, triazoledione may reach concentrations as great as 10 times those of nefazodone, and hence could still be a significant contributor to its therapeutic effects.

Didesmethylsibutramine (BTS-54524) is an active metabolite of the anorectic drug sibutramine that has been identified as an adulterant in weight loss supplements. Data on the activity of didesmethylsibutramine in humans is limited, although a case of psychosis associated with didesmethylsibutramine use was reported in 2019.




















